Risk of Formaldehyde Contamination in Amines from Residual Dichloromethane
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Understanding the mechanism of the formation of impurities in pharmaceutical intermediates and starting materials is crucial for a successful control strategy in the manufacturing of active pharmaceutical ingredients. This paper describes how amines containing residual dichloromethane can form substantial levels of formaldehyde during short-term storage. An investigation involving 22 different amines presents evidence underpinning the role of dichloromethane (DCM) in forming formaldehyde. Additionally, control experiments combined with existing knowledge on the reactivity of DCM with amine nucleophiles provide a mechanistic discussion on the generation of formaldehyde via known adducts from the reaction between amines, dichloromethane, and water. Finally, a case study involving a key intermediate of a drug candidate under investigation at Bristol Myers Squibb demonstrates the impact of residual DCM-derived formaldehyde in amine starting material on the formation of a daughter impurity during an amidation step.
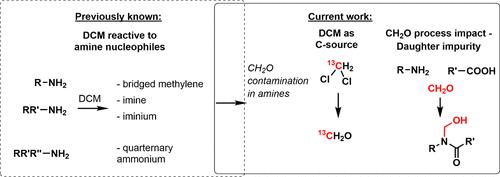
残留二氯甲烷造成胺中甲醛污染的风险
了解医药中间体和起始材料中杂质的形成机理对于在活性药物成分生产过程中成功实施控制策略至关重要。本文介绍了含有残留二氯甲烷的胺如何在短期储存过程中形成大量甲醛。一项涉及 22 种不同胺类的调查提供了二氯甲烷 (DCM) 在形成甲醛过程中所起作用的证据。此外,对照实验结合二氯甲烷与胺亲核物反应的现有知识,对通过胺、二氯甲烷和水反应生成的已知加合物生成甲醛的机理进行了讨论。最后,一项涉及 Bristol Myers Squibb 公司正在研究的候选药物的关键中间体的案例研究表明了胺起始原料中残留的 DCM 衍生甲醛对在酰胺化步骤中形成子杂质的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


