Copper-Catalyzed Oxidative Synthesis of 3-Aryl-5-fluoroalkyl-1,3,4-oxadiazol-2(3H)-ones Using Perfluorocarboxylic Anhydride as a Reagent
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A copper-catalyzed oxidative annulation of sydnones with perfluorocarboxylic anhydride for the synthesis of 3-aryl-5-fluoroalkyl-1,3,4-oxadiazol-2(3H)-ones is developed. A diverse array of 3-aryl-5-fluoroalkyl-1,3,4-oxadiazol-2(3H)-ones are prepared with good yields (>73 examples, yields up to 95%). The synthetic utility of the developed protocol was demonstrated by gram-scale synthesis, and the synthetic transformation to 1,2,4-triazol-3-one products. A mechanistic study suggests that the reaction proceeds via the extrusion of carbon dioxide to generate the hydrazide intermediate, which then undergoes intramolecular cyclization and oxidation.
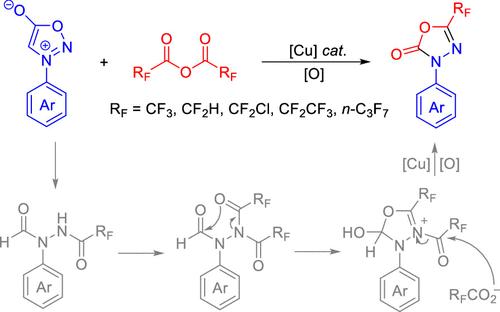
以全氟羧酸酐为试剂,铜催化氧化合成 3-芳基-5-氟烷基-1,3,4-恶二唑-2(3H)-酮
本研究开发了铜催化的全氟羧酸酐氧化环化茚酮法,用于合成 3-芳基-5-氟烷基-1,3,4-恶二唑-2(3H)-酮。制备了多种 3-芳基-5-氟烷基-1,3,4-恶二唑-2(3H)-酮,且收率良好(73 个实例,收率高达 95%)。通过克级合成以及合成转化为 1,2,4-三唑-3-酮产品,证明了所开发方案的合成实用性。机理研究表明,反应是通过二氧化碳挤压生成酰肼中间体,然后进行分子内环化和氧化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


