A Dual CQD-Catalysis and H-Bond Acceptor for Controlling Product Selectivity and Regioselectivity in Symmetric/Unsymmetric Azoxy Arenes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Azoxy arenes are valuable compounds in different areas of chemistry, such as organic chemistry, medicinal chemistry, and natural product chemistry. Despite their value, the regioselective synthesis of unsymmetric azoxybenzenes has remained a real challenge in the field. Herein, the product selectivity in oxidative homocoupling of anilines into symmetric azoxybenzenes was first achieved by an asparagine-functionalized CQD catalyst. Subsequently, in the cross-coupling of anilines into the unsymmetric azoxybenzenes via an ortho H-bond acceptor (HBA) on one of the coupling anilines, the regioselectivity was effectively controlled. It was demonstrated that ortho-HBA could mechanistically establish a six-membered intramolecular hydrogen-bonded ring on an N,N′-dihydroxy intermediate. The formed hydrogen bond makes the nearby nitrogen eminently suitable for the slow dehydration step. As a result, the functional oxygen of the azoxy compound is placed far from the HBA. The o-HBA mechanism also controls the regioselectivity ratio in which 1:0 (with an intramolecular H-bonded hexagonal ring), 2:1 (with an intramolecular H-bonded pentagonal ring), and 1:1 (without an ortho-HBA) isomeric mixtures could be achieved. The HBA mechanism was exploited by different substituted anilines, and various unsymmetric azoxybenzenes were synthesized. Finally, with the aid of mechanistic studies, a plausible mechanism for the reaction was proposed.
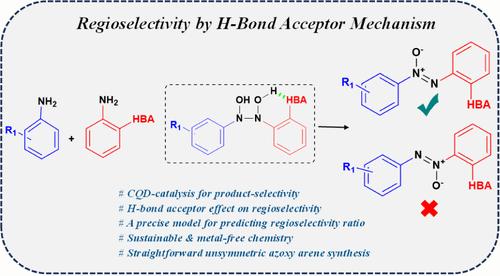
用于控制对称/不对称氮氧烯中产物选择性和区域选择性的双重 CQD 催化和 H 键受体
在有机化学、药物化学和天然产物化学等不同的化学领域,偶氮氧基苯都是非常有价值的化合物。尽管它们具有重要价值,但不对称偶氮氧苯的区域选择性合成仍然是该领域的一个真正挑战。在本文中,首先使用天冬酰胺功能化的 CQD 催化剂实现了苯胺氧化同偶联合成对称氮氧苯的产物选择性。随后,在通过偶联苯胺之一上的正交 H 键受体(HBA)将苯胺与不对称氮氧苯进行交叉偶联时,区域选择性得到了有效控制。研究表明,正交 HBA 可以在 N,N′-二羟基中间体上机械地建立一个六元分子内氢键环。形成的氢键使附近的氮非常适合于缓慢的脱水步骤。因此,叠氮化合物的官能氧远离氢键。o-HBA 机理还控制着区域选择性比例,可以实现 1:0(有分子内 H 键的六角环)、2:1(有分子内 H 键的五角环)和 1:1(没有正 HBA)的异构混合物。不同取代的苯胺利用了 HBA 机理,并合成了各种不对称的偶氮苯。最后,借助机理研究,提出了该反应的合理机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


