Expanding the poly(2-oxazoline) block copolymer possibilities through nitroxide mediated polymerisation†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
In recent years, poly(2-oxazoline)s (POx) have become a sought-after biomaterial to replace PEG. However, access to POx based block copolymers is rather limited and their combination with controlled radical polymerization (CRP) techniques is required. Herein, we report the combination of cationic ring opening polymerization (CROP) and nitroxide mediated radical polymerization (NMP) to enable block copolymerization of poly(2-oxazoline)s with styrenics, acrylics, 1,3-dienes, and acrylamides as the second block. A well-defined poly(2-ethyl-2-oxazoline) macroinitiator has been prepared via CROP and in situ termination via the carboxylic acid functional group of BlocBuilder alkoxyamine has been achieved with a functionalization efficiency of 78%. Four different monomers in each class have been copolymerized via NMP and gel permeation chromatography analysis allowed us to identify the suitable set of comonomers to be utilized in block copolymerization with POx in an efficient, facile, metal- and sulfur-free polymerization environment.
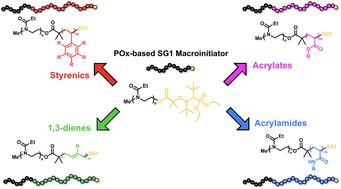
通过氮氧化物介导聚合拓展聚(2-噁唑啉)嵌段共聚物的可能性
近年来,聚(2-噁唑啉)(POx)已成为一种可替代 PEG 的生物材料。然而,基于 POx 的嵌段共聚物的获取途径相当有限,需要将其与受控自由基聚合(CRP)技术相结合。在此,我们报告了阳离子开环聚合(CROP)与硝基氧化物介导自由基聚合(NMP)的结合,以实现聚(2-噁唑啉)与苯乙烯、丙烯酸、1,3-二烯和丙烯酰胺作为第二嵌段的嵌段共聚。通过 CROP 法制备出了定义明确的聚(2-乙基-2-噁唑啉)大引发剂,并通过 BlocBuilder 烷氧基胺的羧酸官能团实现了原位终止,官能化效率高达 78%。通过 NMP 和凝胶渗透色谱分析,我们确定了在高效、简便、不含金属和硫的聚合环境中与 POx 进行嵌段共聚的合适共聚单体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


