Strengthened Delocalized Electronic Effect in Nano-Nickel@Carbon with High Pyrrolic Nitrogen for Selective Hydrogenation of Substituted Nitrobenzene Hydrogenation
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Carbon-encapsulated metal (CEM) catalysts reconfigure the active site of the catalytic reaction by shifting from the conventional metal to the surface of the carbon material. Carbon-encapsulated structure has attracted wide attention in the fields of electrochemistry, thermal catalysis and photocatalysis. Herein, a nitrogen-doped carbon-encapsulated nickel catalyst was synthesized via hydrothermal synthesis, with pyrrolic N (NPyr) content accounting for 48.4% of the total nitrogen species. Experiments and density functional theory calculations reveal that the five-membered pyrrole ring shares six π electrons, and its electron cloud density on the carbon surface surpasses that of benzene or pyridine ring, promoting extensive electronic interaction between NPyrC and nickel. The interaction also extends beyond the vicinity of the doping sites and permeates throughout the entire carbon shell, thereby augmenting a greater number of potential active sites on the NC layer. This strengthened delocalized electronic effect imparts specificity in the adsorption and dissociation processes of hydrogen and p-chloronitrobenzene, leading to enhanced catalytic performance in the hydrogenation production of p-chloroaniline. The precise preparation of NPyr-doped CEM catalysts demonstrates its huge potential for industrial applications.
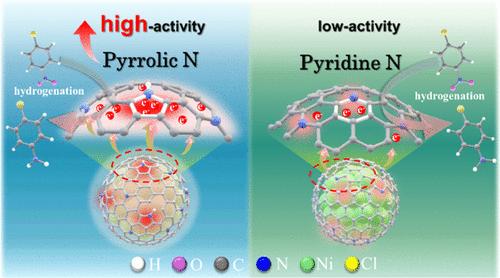
具有高吡咯烷酮氮的纳米镍@碳中用于取代硝基苯选择性加氢的强化去局域电子效应
碳包覆金属(CEM)催化剂通过从传统金属转移到碳材料表面,重新配置了催化反应的活性位点。碳包覆结构在电化学、热催化和光催化领域引起了广泛关注。本文通过水热合成法合成了一种掺氮碳包封镍催化剂,其中吡咯烷酮氮(NPyr)含量占总氮量的 48.4%。实验和密度泛函理论计算显示,五元吡咯环共享六个π电子,其在碳表面的电子云密度超过了苯环或吡啶环,促进了 NPyrC 与镍之间广泛的电子相互作用。这种相互作用还超越了掺杂位点附近的范围,渗透到整个碳壳,从而增加了 NC 层上潜在活性位点的数量。这种强化的局部电子效应使氢和对氯硝基苯的吸附和解离过程具有特异性,从而提高了对氯苯胺氢化生产的催化性能。掺杂 NPyr 的 CEM 催化剂的精确制备证明了其在工业应用中的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


