AARS1 and AARS2 sense l-lactate to regulate cGAS as global lysine lactyltransferases
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
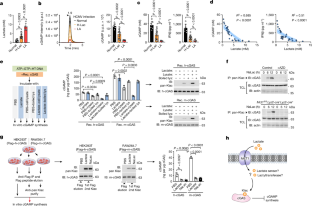
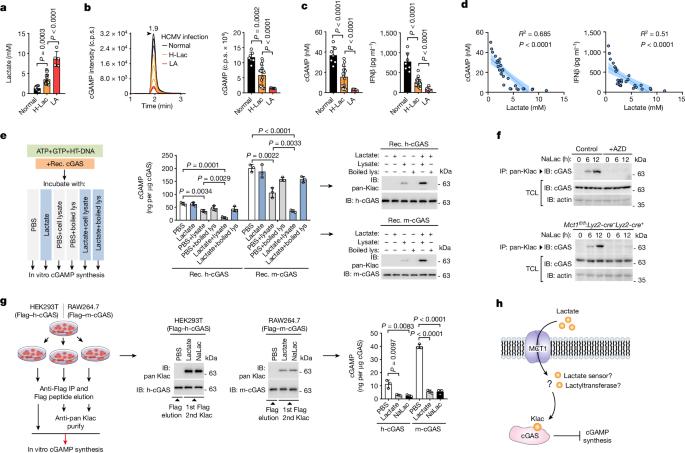
l-lactate modifies proteins through lactylation1, but how this process occurs is unclear. Here we identify the alanyl-tRNA synthetases AARS1 and AARS2 (AARS1/2) as intracellular l-lactate sensors required for l-lactate to stimulate the lysine lactylome in cells. AARS1/2 and the evolutionarily conserved Escherichia coli orthologue AlaRS bind to l-lactate with micromolar affinity and they directly catalyse l-lactate for ATP-dependent lactylation on the lysine acceptor end. In response to l-lactate, AARS2 associates with cyclic GMP–AMP synthase (cGAS) and mediates its lactylation and inactivation in cells and in mice. By establishing a genetic code expansion orthogonal system for lactyl-lysine incorporation, we demonstrate that the presence of a lactyl moiety at a specific cGAS amino-terminal site abolishes cGAS liquid-like phase separation and DNA sensing in vitro and in vivo. A lactyl mimetic knock-in inhibits cGAS, whereas a lactyl-resistant knock-in protects mice against innate immune evasion induced through high levels of l-lactate. MCT1 blockade inhibits cGAS lactylation in stressed mice and restores innate immune surveillance, which in turn antagonizes viral replication. Thus, AARS1/2 are conserved intracellular l-lactate sensors and have an essential role as lactyltransferases. Moreover, a chemical reaction process of lactylation targets and inactivates cGAS. The tRNA synthases AARS1 and AARS2 are identified as evolutionarily conserved sensors of intracellular l-lactate to mediate the global lysine lactylome.
AARS1 和 AARS2 感知乳酸以调节作为全局赖氨酸乳酰转移酶的 cGAS
乳酸通过乳化作用修饰蛋白质1,但这一过程是如何发生的尚不清楚。在这里,我们发现丙氨酰-tRNA 合成酶 AARS1 和 AARS2(AARS1/2)是细胞内的乳酸传感器,它们是乳酸刺激细胞内赖氨酸乳化组所必需的。AARS1/2 和进化保守的大肠杆菌直向同源物 AlaRS 能以微摩尔的亲和力与乳酸结合,并直接催化乳酸在赖氨酸接受端进行 ATP 依赖性乳化。在反应 l-乳酸时,AARS2 与环 GMP-AMP 合成酶(cGAS)结合,并在细胞和小鼠体内介导其乳化和失活。通过建立乳酰-赖氨酸结合的遗传密码扩增正交系统,我们证明了在特定的 cGAS 氨基末端位点存在乳酰分子会取消 cGAS 在体外和体内的液相分离和 DNA 传感。乳基模拟基因敲入抑制了cGAS,而乳基抗性基因敲入保护了小鼠免受高水平l-乳酸盐诱导的先天性免疫逃避。阻断 MCT1 可抑制受压小鼠的 cGAS 乳化,恢复先天免疫监视,进而抑制病毒复制。因此,AARS1/2是细胞内保守的l-乳酸传感器,作为乳酰转移酶发挥着重要作用。此外,乳酸化的化学反应过程以 cGAS 为目标并使其失活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


