Nature of Glucose Epimerization Catalyzed by Mo-Containing Bulk Catalysts in Aqueous Phase
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
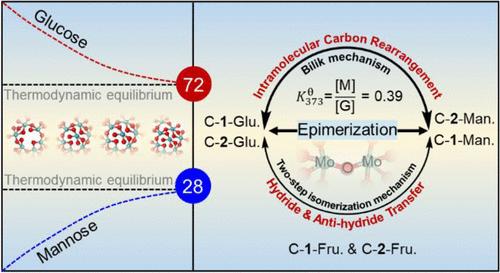
The nature of Mo-catalyzed glucose epimerization in the aqueous phase was elaborately studied. We herein formulate the thermodynamic properties (e.g., ΔrHT, ΔrGT, and Keq.T) of the reversible epimerization by collecting the equilibrium composition. The isotopic tracing and NMR spectra show that the overall tautomerization network encompasses the reversible epimerization and isomerization and the irreversible degradation of all hexoses. The leaching tests and kinetic and spectroscopic studies reveal that glucose epimerization catalyzed by Mo-containing solid catalysts in the aqueous phase resembles homogeneous catalysis. All catalysts enable a near-equilibrium yield of mannose (28%) at 373 K except MoP but undergo a different kinetic course of which MoN is the best catalyst according to the apparent kinetic parameters. The molybdenum species dissolved in an aqueous solution evolves into the truly active centers of the MoVI–O–MoVI bridged polymolybdates. Moreover, we propose that a single Mo center as Lewis acidic site coordinates with the aldoses to form a bidentate complex, which thereby contributes two different mechanisms to generate the epimers, viz., the intramolecular 1, 2-carbon exchange and two-step isomerization. The former proceeds through a three-membered cyclic transition state (TSC) that mediates the simultaneous cleavage of the bond between C-2 and C-3 and formation of the bond between C-1 and C-3, whereas the latter undergoes two hydride transition states (TSH-1 and TSH-2) via the hydride transfer twice, leading to the chiral inversion of the configuration at C-2. Last but not least, the presence of phosphates in an aqueous solution leads to the deactivation of Mo-based catalysts because of the interplay between glucose and phosphates.
含钼块状催化剂在水相催化葡萄糖外延的性质
我们详细研究了水相中钼催化葡萄糖缩合反应的性质。在此,我们通过收集平衡组成,提出了可逆表聚的热力学性质(如 ΔrHT、ΔrGT 和 Keq.T)。同位素示踪和核磁共振光谱显示,整个同分异构网络包括所有己糖的可逆表聚和异构化以及不可逆降解。浸出试验以及动力学和光谱研究表明,含钼固体催化剂在水相催化的葡萄糖表聚类似于均相催化。在 373 K 时,除 MoP 外,所有催化剂都能使甘露糖的产率接近平衡产率(28%),但其动力学过程各不相同,根据表观动力学参数,MoN 是最佳催化剂。溶解在水溶液中的钼物种演变成了 MoVI-O-MoVI 桥接多钼酸盐的真正活性中心。此外,我们还提出,作为路易斯酸性位点的单个钼中心与醛糖配位形成双齿配合物,从而产生两种不同的机制来生成表聚物,即分子内 1、2 碳交换和两步异构化。前者通过一个三元环状转变态(TSC)同时裂解 C-2 和 C-3 之间的键并形成 C-1 和 C-3 之间的键,而后者则通过两次氢化物转移经历两个氢化物转变态(TSH-1 和 TSH-2),导致 C-2 的构型发生手性反转。最后但并非最不重要的一点是,由于葡萄糖和磷酸盐之间的相互作用,水溶液中磷酸盐的存在会导致钼基催化剂失活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


