Enhanced foam fractionation of perfluorooctane sulfonate (PFOS) from water using amphiphilic Janus SiO2 nanoparticles
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
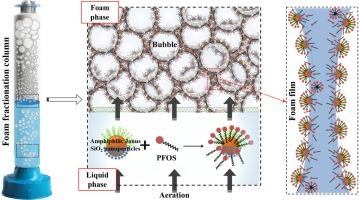
Perfluorooctane sulfonate (PFOS), a persistent environmental contaminant, presents significant risks to human health and ecological balance. In response, our research introduces an innovative approach to tackle this challenge through the development of an efficient nanoparticle surfactant that enhances PFOS removal via foam fractionation. We synthesized a novel amphiphilic Janus nanoparticle (F-SNP-NH2) by chemically modifying SiO2 nanoparticles with 1H,1H,2H,2H-perfluorooctyltrimethoxysilane and aminopropyltriethoxysilane, employing an interface masking strategy. This unique particle, bearing hydrophobic fluorocarbon chains and hydrophilic amino groups, offers surface properties conducive to foam fractionation. We verified the structure and chemical composition of F-SNP-NH2 through SEM-EDX, FTIR, and XPS. Its high surface activity enabled effective adsorption at the air–water interface, thereby promoting foam stability. Correspondingly, the foam height and half-life reached 24.5 mm and 316 s, respectively. Significantly, F-SNP-NH2 reached PFOS adsorption equilibrium in a short duration of 40 min, achieving a maximum adsorption capacity of 1015.04 mg/g. Our analysis, supported by experimental data, theoretical models, and DFT calculations, pinpointed F–F and hydrophobic interactions as the primary forces driving PFOS collection. Under suitable conditions for foam fractionation, specifically at pH 5.0 and air flowrate 700 mL/min, the removal efficiency of PFOS achieved a high range of 98.8 % to 99.2 %, with corresponding enrichment ratios varying from 23.6 to 24.9. The foam fractionation process, employing F-SNP-NH2, demonstrated high selectivity and efficiency for PFOS removal, showcased the reusability of the nanoparticle surfactant. These results highlight the potential of F-SNP-NH2 in advancing foam fractionation technology for the targeted removal of PFOS and other per- and polyfluoroalkyl substances.
利用两亲性 Janus SiO2 纳米粒子加强泡沫分馏水中的全氟辛烷磺酸 (PFOS)
全氟辛烷磺酸(PFOS)是一种持久性环境污染物,对人类健康和生态平衡构成重大风险。为此,我们的研究引入了一种创新方法来应对这一挑战,即开发一种高效的纳米粒子表面活性剂,通过泡沫分馏提高全氟辛烷磺酸的去除率。我们采用界面掩蔽策略,用 1H,1H,2H,2H-全氟辛基三甲氧基硅烷和氨丙基三乙氧基硅烷对二氧化硅纳米粒子进行化学修饰,合成了一种新型两亲性 Janus 纳米粒子(F-SNP-NH2)。这种独特的颗粒带有疏水性碳氟化合物链和亲水性氨基,具有有利于泡沫分馏的表面特性。我们通过 SEM-EDX、FTIR 和 XPS 验证了 F-SNP-NH2 的结构和化学成分。F-SNP-NH2的高表面活性使其能在空气-水界面上有效吸附,从而提高了泡沫的稳定性。相应地,泡沫高度和半衰期分别达到了 24.5 毫米和 316 秒。值得注意的是,F-SNP-NH2 在短短的 40 分钟内就达到了全氟辛烷磺酸的吸附平衡,最大吸附容量为 1015.04 mg/g。我们的分析得到了实验数据、理论模型和 DFT 计算的支持,指出 F-F 和疏水相互作用是驱动 PFOS 收集的主要力量。在合适的泡沫分馏条件下,特别是在 pH 值为 5.0 和空气流量为 700 mL/min 的条件下,全氟辛烷磺酸的去除率高达 98.8 % 至 99.2 %,相应的富集比为 23.6 至 24.9。采用 F-SNP-NH2 的泡沫分馏过程显示出了去除全氟辛烷磺酸的高选择性和高效性,展示了纳米表面活性剂的可重复使用性。这些结果凸显了 F-SNP-NH2 在推动泡沫分馏技术定向去除全氟辛烷磺酸及其他全氟和多氟烷基物质方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


