Development of a genome engineering tool for insertion of pathway-sized DNAs in Escherichia coli
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-09-24
DOI:10.1016/j.jtice.2024.105776
引用次数: 0
Abstract
Background
The approach of metabolic engineering enables reprogramming of the producer cell to overproduce products of interest. It usually requires simultaneous manipulation of many genes to achieve the engineering purpose. To circumvent the plasmid-incurred problems, the recombineering technology mainly involving λ Red has been developed for genomic insertion of target genes. However, these λ Red-dependent recombination methods are generally afflicted by insertion of a large size DNA construct. This issue was addressed by development of a genome engineering tool by combination of phage integrases with CRISPR-λ (PIC herein).
Methods
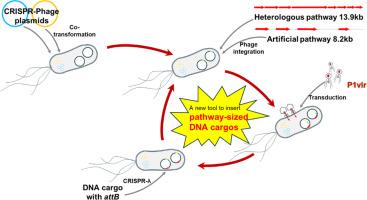
As a proof of concept, PIC was applied for engineering of metabolic pathways leading to pyridine nucleotides. This was carried out by assembly of a heterologous operon (13.9 kb) and an artificial operon (8.2 kb) composed of endogenous genes. Aided by phage integrases, the two assembled DNA constructs were sequentially integrated into the prophage attB sites of Escherichia coli at high efficiency. The kanamycin cassette from the Keio strain collection was leveraged by CRISPR-λ to eliminate the undesired gene in E. coli by knock-in of the attB site which was utilized for iterative integration of the DNA construct.
Significant findings
The engineered strain consequently overproduced uracil. It resulted in a 160-fold increase in uracil over that of the parent strain. This evidently suggests that the producer strain harbors reprogrammed metabolic pathways in favor of the uracil synthesis. Overall, the result indicates that the developed system is useful for metabolic engineering of E. coli by genomic insertion of pathway-sized DNA cargos.
开发用于在大肠杆菌中插入路径大小 DNA 的基因组工程工具
背景 新陈代谢工程方法可以对生产细胞进行重编程,使其过度生产感兴趣的产品。它通常需要同时操作多个基因才能达到工程目的。为了避免质粒引起的问题,人们开发了主要涉及 λ Red 的重组工程技术,用于目标基因的基因组插入。然而,这些依赖于 λ Red 的重组方法通常会受到插入大尺寸 DNA 构建体的影响。作为概念验证,PIC 被应用于吡啶核苷酸代谢途径的工程化。其方法是组装一个异源操作子(13.9 kb)和一个由内源基因组成的人工操作子(8.2 kb)。在噬菌体整合酶的帮助下,两个组装好的 DNA 构建体依次高效整合到大肠杆菌的噬菌体 attB 位点上。CRISPR-λ利用庆应义塾菌株中的卡那霉素盒,通过敲入attB位点来消除大肠杆菌中不需要的基因,并利用attB位点迭代整合DNA构建体。与亲本菌株相比,尿嘧啶的产量增加了 160 倍。这显然表明,生产菌株的代谢途径经过重新编程,有利于尿嘧啶的合成。总之,这一结果表明,所开发的系统有助于通过在基因组中插入通路大小的 DNA 货物来实现大肠杆菌的代谢工程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


