Simvastatin induces ferroptosis and activates anti-tumor immunity to sensitize anti-PD-1 immunotherapy in microsatellite stable gastric cancer
IF 4.8
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Background
Gastric cancer (GC), especially the case with microsatellite stability (MSS) phenotype, has limited efficacy for immune checkpoint blockade (ICB) therapy. Metabolism reprogramming is newly recognized to affect tumor immune microenvironment (TIME). However, the relationship between metabolism reprogramming and immunotherapy for MSS GC has not been reported.
Methods
A metabolic stratification for GC was developed based on the glycolysis/cholesterol synthesis axis using the R package “ConsensusClusterPlus”. The T cell inflamed score was used to define “immune-hot” and “immune-cold” phenotypes in MSS GC. The anti-tumor and immunological effects of simvastatin were explored using in vitro and in vivo experiments.
Results
Three metabolic subtypes were identified in GC patients, including cholesterol, glycolysis and quiescent subtypes. The cholesterol subtype was associated with poorer clinical features and higher tumor purity. Correspondingly, we demonstrated that simvastatin, a specific inhibitor of cholesterol synthesis, significantly inhibited the proliferation, migration, and induced ferroptosis in GC cells. Interestingly, simvastatin markedly inhibited tumor growth in immunocompetent mice, while no significant effect in immunodeficient mice. Upregulation of chemokines and increased recruitment of CD8+ T cells were observed after simvastatin treatment. Consistently, the cholesterol subtype exhibited a less inflamed TIME and coincided significantly with the “immune-cold” phenotype of MSS GC. Finally, we confirmed simvastatin enhanced PD-1 blockade efficacy via modulating the TIME and activating anti-tumor immunity in tumor-bearing mice.
Conclusion
Our data revealed the significance of cholesterol synthesis in GC and demonstrated simvastatin served as a promising sensitizer for ICB therapy by inducing ferroptosis and anti-tumor immunity in MSS GC patients.
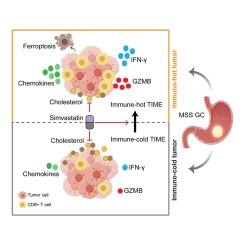
辛伐他汀诱导铁蛋白沉积,激活抗肿瘤免疫,使抗PD-1免疫疗法对微卫星稳定型胃癌敏感
背景胃癌(GC),尤其是具有微卫星稳定性(MSS)表型的胃癌,对免疫检查点阻断(ICB)疗法的疗效有限。新陈代谢重编程被认为会影响肿瘤免疫微环境(TIME)。方法:使用 R 软件包 "ConsensusClusterPlus",基于糖酵解/胆固醇合成轴对 GC 进行代谢分层。用 T 细胞炎症评分来定义 MSS GC 的 "免疫热 "和 "免疫冷 "表型。结果在 GC 患者中发现了三种代谢亚型,包括胆固醇亚型、糖酵解亚型和静息亚型。胆固醇亚型与较差的临床特征和较高的肿瘤纯度有关。相应地,我们证实辛伐他汀作为胆固醇合成的特异性抑制剂,能显著抑制 GC 细胞的增殖、迁移并诱导铁变态反应。有趣的是,辛伐他汀能明显抑制免疫功能正常小鼠的肿瘤生长,而对免疫缺陷小鼠则无明显影响。辛伐他汀治疗后,可观察到趋化因子的上调和 CD8+ T 细胞的招募增加。同样,胆固醇亚型的 TIME 炎症较轻,与 MSS GC 的 "免疫冷 "表型明显吻合。结论我们的数据揭示了胆固醇合成在 GC 中的重要作用,并证明辛伐他汀通过诱导 MSS GC 患者的铁变态反应和抗肿瘤免疫,可作为 ICB 治疗的增敏剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


