New ruthenium(II) isocyanide catalysts for the transfer hydrogenation of ethyl levulinate to γ-valerolactone in C2-C6 alcohols
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Transfer hydrogenation (TH) processes are receiving great attention for biomass valorization and ruthenium(II) complexes are renowned TH catalysts both on laboratory and industrial scale. Only a few homogeneous catalytic precursors are available in the literature for the TH of ethyl levulinate (EL) to γ-valerolactone (GVL). Herein, starting from simple, commercially available isocyanides, two classes of air-stable ruthenium(II) complexes were synthesized and tested as catalytic precursors. First, an optimized preparation of Ru(II) p-cymene isocyanide complexes was developed. Then, the thermally induced p-cymene/DMSO substitution gave access to unprecedented ruthenium isocyanide-DMSO complexes. All the complexes were characterized and tested in TH of EL to GVL showing promising performances, adopting 2-propanol as hydrogen donor, a low catalyst (Ru) and co-catalyst (KOH) amount, working under microwave heating for 1 h at 150 °C. The most selective systems were also successfully tested with different biomass-derived alcohols, including 2-butanol. Finally, the recycling of the best catalyst was also investigated, thus improving the efficiency of the entire process.
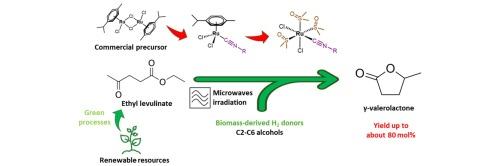
在 C2-C6 脂肪醇中将乙酰丙酸乙酯转移氢化为 γ-戊内酯的新型异氰化钌催化剂
转移加氢(TH)过程在生物质能值化方面受到极大关注,而钌(II)络合物是实验室和工业规模的著名 TH 催化剂。文献中只有少数均相催化前体可用于将乙酰丙酸乙酯(EL)转化为γ-戊内酯(GVL)。在此,我们从简单的市售异氰酸酯开始,合成了两类空气稳定的钌(II)配合物,并将其作为催化前体进行了测试。首先,开发出了对伞花烃异氰酸钌(II)络合物的优化制备方法。然后,通过热诱导对亚甲基/二甲基亚砜的取代,获得了前所未有的异氰酸钌-二甲基亚砜配合物。采用 2-丙醇作为氢供体,低催化剂(Ru)和助催化剂(KOH)用量,在 150 °C 下微波加热 1 小时,所有配合物在 EL 至 GVL 的 TH 反应中均表现出良好的性能。选择性最强的系统还成功地对不同的生物质衍生醇(包括 2-丁醇)进行了测试。最后,还对最佳催化剂的回收利用进行了研究,从而提高了整个工艺的效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


