Personalized PDAC chip with functional endothelial barrier for tumour biomarker detection: A platform for precision medicine applications
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer characterised by poor survival rates and an increasing global incidence. Advances in the staging and categorization of pancreatic tumours, along with the discovery of functional mutations, have made precision treatments possible, which may lead to better clinical results. To further improve customized treatment approaches, in vitro models that can be used for functional drug sensitivity testing and precisely mimic the disease at the organ level are required. In this study, we present a workflow for creating a personalized PDAC chip utilising primary tumour-derived human pancreatic organoids (hPOs) and Human Umbilical Vein Endothelial Cells (HUVECs) to simulate the vascular barrier and tumour interactions within a PDMS-free organ-on-a-chip system. The patient PDAC tissue, expanded as tumour hPOs, could be cultured as adherent cells on the chip for more than 50 days, allowing continuous monitoring of cell viability through outflows from tumour and endothelial channels. Our findings demonstrate a gradual increase in cell density and cell turnover in the pancreatic tumor channel. Tumour-specific biomarkers, including CA-19.9, TIMP-1, Osteopontin, MIC-1, ICAM-1 and sAXL were consistently detected in the PDAC chip outflows. Comparative analyses between tissue culture plates and microfluidic conditions revealed significant differences in biomarker secretion patterns, highlighting the advantages of the microfluidics approach. This PDAC chip provides a stable, reproducible tumour model system with a functional endothelial cell barrier, suitable for drug sensitivity and secretory biomarker studies, thus serving as a platform for functional precision medicine application and multi-organ chip development.
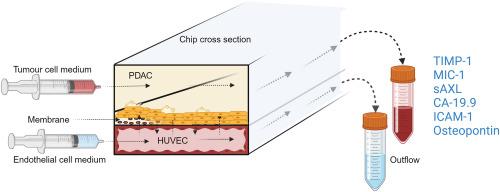
用于肿瘤生物标记物检测的具有功能性内皮屏障的个性化 PDAC 芯片:精准医疗应用平台
胰腺导管腺癌(PDAC)是一种侵袭性很强的癌症,其特点是生存率低,全球发病率不断上升。胰腺肿瘤分期和分类方面的进展以及功能性突变的发现使精准治疗成为可能,这可能会带来更好的临床效果。为进一步改善定制化治疗方法,需要可用于功能性药物敏感性测试并在器官水平上精确模拟疾病的体外模型。在本研究中,我们介绍了一种创建个性化 PDAC 芯片的工作流程,利用原发肿瘤衍生的人胰腺器官组织(hPO)和人脐静脉内皮细胞(HUVEC)在不含 PDMS 的器官芯片系统中模拟血管屏障和肿瘤相互作用。作为肿瘤 hPOs 扩增的 PDAC 患者组织可以作为粘附细胞在芯片上培养 50 多天,从而可以通过肿瘤和内皮通道流出的细胞持续监测细胞活力。我们的研究结果表明,胰腺肿瘤通道中的细胞密度和细胞周转率逐渐增加。在 PDAC 芯片流出物中持续检测到肿瘤特异性生物标记物,包括 CA-19.9、TIMP-1、Osteopontin、MIC-1、ICAM-1 和 sAXL。组织培养板和微流控条件下的对比分析表明,生物标志物的分泌模式存在显著差异,这凸显了微流控方法的优势。该 PDAC 芯片提供了一个稳定、可重复的肿瘤模型系统,具有功能性内皮细胞屏障,适合药物敏感性和分泌型生物标记物研究,因此可作为功能性精准医学应用和多器官芯片开发的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


