Palladium-catalyzed desymmetric coupling reaction between silacyclobutanes and terminal alkynes for the synthesis of silicon-stereogenic allyl vinylsilanes
引用次数: 0
Abstract
A palladium/TADDOL-derived phosphonate catalyzed desymmetric ring-opening coupling bewteen prochiral silacyclobutanes and alkyl terminal alkynes was developed. This catalytic system facilitates the formation of optically active allyl vinylsilane compounds featuring a quaternary silicon-stereogenic center, achieving good to high yields and moderate to good enantiomeric ratios. This approach significantly broaden the scope of ring-opening desymmetrization reactions involving silacyclobutanes under transition metal catalysis.
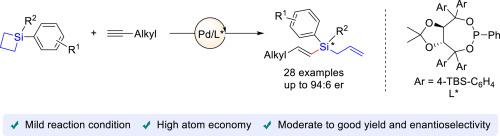
钯催化硅环丁烷与末端炔烃的不对称偶联反应,用于合成硅稳定烯丙基乙烯基硅烷
本研究开发了一种由钯/TADDOL衍生的膦酸盐催化的非对称开环偶联反应,该反应涉及手性硅环丁烷和烷基末端炔。这种催化体系有助于形成具有光学活性的烯丙基乙烯基硅烷化合物,其特征是具有一个季硅烷中心,可获得良好到较高的产率和中等到较好的对映异构比。这种方法大大拓宽了过渡金属催化下涉及硅环丁烷的开环不对称反应的范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


