Inorganic porous carbon as the supporter of H2TiO3 via in-situ polymerization synchronous conversion for lithium recovery from aqueous solutions
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The extraction of lithium from liquid minerals, such as salt lake brines and underground brines, has garnered extensive interest due to its eco-friendly and cost-effective properties. However, the effectiveness of this method is constrained by the stability and capacity of the materials. Herein, a new-type inorganic carbon-supported H2TiO3 adsorbent was developed by a novel in-situ polymerization synchronous conversion strategy. It was found that when resorcinol and formaldehyde containing Li2CO3 and TiO2 were polymerized in-situ and then calcined at 973.15 K, the resin was successfully carbonized to obtain the carbon supporter with a low degree of disorder, and the Li2CO3 and TiO2 in the supporter were synchronously converted into Li2TiO3 and uniformly dispersed in the carbon matrix. Because of the large specific surface area and strong hydrophilicity of the carbon supporter, the material exhibited a maximum Li+ adsorption capacity of 52.14 mg·g−1, which was far higher than that of inorganic composite materials reported at present. Even the equilibrium adsorption capacity for Li+ at a low concentration of 29.26 mg·L−1 reached 28.51 mg·g−1. Following adsorption, the Li+ in the material was easily eluted by 0.25 mol·L−1 HCl, and the elution rate was more than 90 % within 2 h. Dynamic adsorption using a fixed-bed was also performed at 298.15 and 343.15 K, and the adsorption capacity for the same concentration of Li+ was 7.08 and 9.44 mg·g−1, respectively. Because of the high capacity for low-concentration Li+ and the promoting effect of temperature on adsorption, the material is well-suited for recovering Li+ from liquid resources, particularly from geothermal water with high temperatures and low Li+ concentrations.
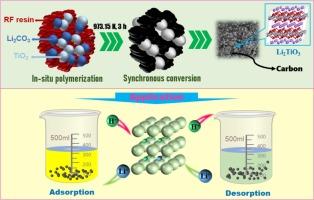
无机多孔碳作为 H2TiO3 的支撑剂,通过原位聚合同步转化从水溶液中回收锂
从盐湖卤水和地下卤水等液态矿物中提取锂,因其环保和成本效益高的特性而受到广泛关注。然而,这种方法的有效性受到材料稳定性和容量的限制。本文采用新型原位聚合同步转化策略,开发了一种新型无机碳支撑 H2TiO3 吸附剂。研究发现,将含有Li2CO3和TiO2的间苯二酚和甲醛原位聚合,然后在973.15 K下煅烧,树脂被成功碳化,得到无序度较低的碳支撑体,支撑体中的Li2CO3和TiO2同步转化为Li2TiO3,并均匀地分散在碳基体中。由于碳支撑体具有较大的比表面积和较强的亲水性,该材料对 Li+ 的最大吸附容量为 52.14 mg-g-1,远高于目前报道的无机复合材料。即使在 29.26 mg-L-1 的低浓度下,该材料对 Li+ 的平衡吸附容量也达到了 28.51 mg-g-1。在 298.15 和 343.15 K 的条件下,还使用固定床进行了动态吸附,对相同浓度的 Li+ 的吸附容量分别为 7.08 和 9.44 mg-g-1。由于对低浓度 Li+ 的高吸附容量以及温度对吸附的促进作用,该材料非常适合从液体资源中回收 Li+,特别是从高温和低浓度 Li+ 的地热水中回收 Li+。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


