A Supramolecular Nanoformulation with Adaptive Photothermal/Photodynamic Transformation for Preventing Dental Caries
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
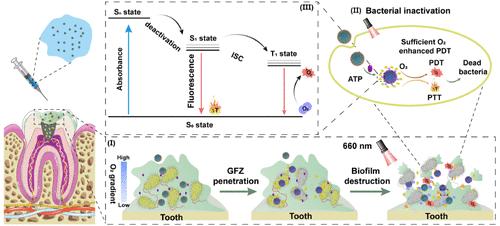
In the context of an increasingly escalating antibiotics crisis, phototherapy has emerged as a promising therapeutic approach due to its inherent advantages, including high selectivity, noninvasiveness, and low drug resistance. Photothermal therapy (PTT) and photodynamic therapy (PDT) are two complementary and promising phototherapies albeit with inherent limitations, noted as the challenges in achieving precise heat confinement and the associated risk of off-target damage for PTT, while the constraints due to the hypoxic microenvironment are prevalent in biofilms faced by PDT. Herein, we have designed a supramolecular nanoformulation that leverages the complexation-induced quenching of guanidinium-modified calix[5]arene grafted with fluorocarbon chains (GC5AF5), the efficient recognition of adenosine triphosphate (ATP), and the oxygen-carrying capacity of the fluorocarbon chain. This intelligent nanoformulation enables the adaptive enhancement of both photothermal therapy (PTT) and photodynamic therapy (PDT), allowing for on-demand switching between the two modalities. Our nanoformulation utilizes ATP released by dead bacteria to accelerate the elimination of biofilms, rendering bacteria unable to resist while minimizing harm to healthy tissues. This research highlights the particular recognition and assembly capabilities of macrocycles, offering a promising strategy for creating potent, combined antibiofilm therapies.
用于预防龋齿的具有自适应光热/光动力转化功能的超分子纳米制剂
在抗生素危机日益加剧的背景下,光疗因其固有的优势,包括高选择性、非侵入性和低耐药性,已成为一种前景广阔的治疗方法。光热疗法(PTT)和光动力疗法(PDT)是两种互补且前景广阔的光疗方法,但也有其固有的局限性,PTT 面临着实现精确热约束的挑战和相关的脱靶损伤风险,而 PDT 则面临着生物膜中普遍存在的缺氧微环境的限制。在此,我们设计了一种超分子纳米制剂,利用了接枝了碳氟化合物链(GC5AF5)的胍修饰钙[5]炔的络合诱导淬灭、三磷酸腺苷(ATP)的高效识别以及碳氟化合物链的携氧能力。这种智能纳米制剂能够自适应地增强光热疗法(PTT)和光动力疗法(PDT),实现两种疗法的按需切换。我们的纳米制剂利用死亡细菌释放的 ATP 加速消除生物膜,使细菌无法抵抗,同时将对健康组织的伤害降至最低。这项研究凸显了大环的特殊识别和组装能力,为创造强效的联合抗生物膜疗法提供了一种前景广阔的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


