Defining heritability, plasticity, and transition dynamics of cellular phenotypes in somatic evolution
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
Single-cell sequencing has characterized cell state heterogeneity across diverse healthy and malignant tissues. However, the plasticity or heritability of these cell states remains largely unknown. To address this, we introduce PATH (phylogenetic analysis of trait heritability), a framework to quantify cell state heritability versus plasticity and infer cell state transition and proliferation dynamics from single-cell lineage tracing data. Applying PATH to a mouse model of pancreatic cancer, we observed heritability at the ends of the epithelial-to-mesenchymal transition spectrum, with higher plasticity at more intermediate states. In primary glioblastoma, we identified bidirectional transitions between stem- and mesenchymal-like cells, which use the astrocyte-like state as an intermediary. Finally, we reconstructed a phylogeny from single-cell whole-genome sequencing in B cell acute lymphoblastic leukemia and delineated the heritability of B cell differentiation states linked with genetic drivers. Altogether, PATH replaces qualitative conceptions of plasticity with quantitative measures, offering a framework to study somatic evolution. Phylogenetic analysis of trait heritability (PATH) applies phylogenetic correlations to single-cell lineage tracing data, quantifying cell state plasticity and transition probabilities. PATH offers insights into cell state heritability and transition dynamics in cancers.
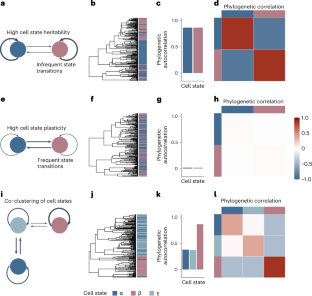
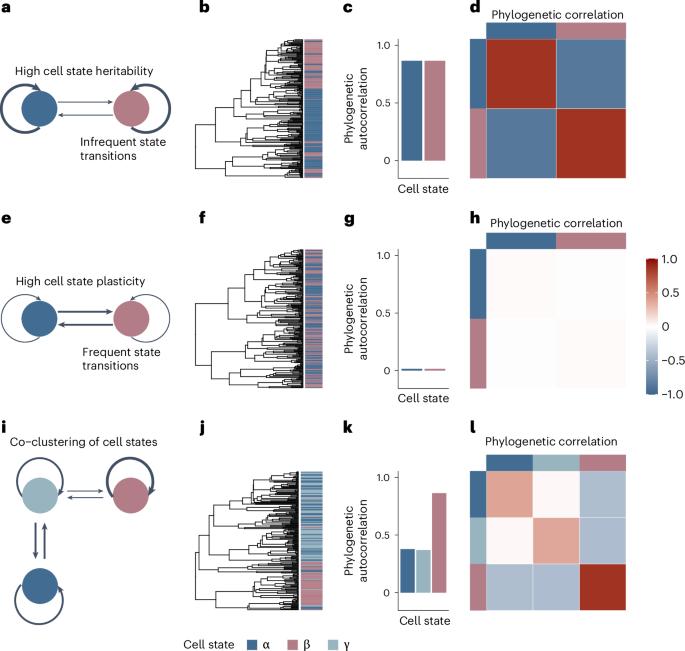
定义体细胞进化过程中细胞表型的遗传性、可塑性和过渡动力学
单细胞测序技术描述了各种健康和恶性组织的细胞状态异质性。然而,这些细胞状态的可塑性或遗传性在很大程度上仍然未知。为了解决这个问题,我们引入了 PATH(性状遗传性系统发育分析),这是一个量化细胞状态遗传性与可塑性的框架,可以从单细胞系追踪数据推断细胞状态的转变和增殖动态。我们将 PATH 应用于小鼠胰腺癌模型,观察到上皮细胞向间质细胞转变谱两端的遗传性,而中间状态的可塑性更高。在原发性胶质母细胞瘤中,我们发现了干细胞和间充质样细胞之间的双向转变,这种转变以星形胶质细胞样状态为中介。最后,我们从 B 细胞急性淋巴细胞白血病的单细胞全基因组测序中重建了系统发育,并描述了与遗传驱动因素相关的 B 细胞分化状态的遗传性。总之,PATH 以定量测量取代了可塑性的定性概念,为研究体细胞进化提供了一个框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


