Simultaneous Photocatalytic Production of H2 and Acetal from Ethanol with Quantum Efficiency over 73% by Protonated Poly(heptazine imide) under Visible Light
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In this work, protonated poly(heptazine imide) (H-PHI) was obtained by adding acid to the suspension of potassium PHI (K-PHI) in ethanol. It was established that the obtained H-PHI demonstrates very high photocatalytic activity in the reaction of hydrogen formation from ethanol in the presence of Pt nanoparticles under visible light irradiation in comparison with K-PHI. This enhancement can be attributed to improved efficiency of photogenerated charge transfer to the photocatalyst’s surface, where redox processes occur. Various factors influencing the system’s activity were evaluated. Notably, it was discovered that the conditions of acid introduction into the system can significantly affect the size of Pt (cocatalyst metal) deposition on the H-PHI surface, thereby enhancing the photocatalytic system’s stability in producing molecular hydrogen. It was established that the system can operate efficiently in the presence of air without additional components on the photocatalyst surface to block air access. Under optimal conditions, the apparent quantum yield of molecular hydrogen production at 410 nm is around 73%, the highest reported value for carbon nitride materials to date. The addition of acid not only increases the activity of the reduction part of the system but also leads to the formation of a value-added product from ethanol–1,1-diethoxyethane (acetal) with high selectivity.
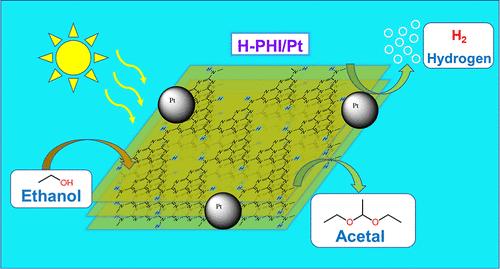
质子化聚(庚嗪亚胺)在可见光下同时光催化生产乙醇中的 H2 和乙缩醛,量子效率超过 73
在这项研究中,通过在乙醇中的 PHI 钾(K-PHI)悬浮液中加入酸,得到了质子化聚(庚嗪亚胺)(H-PHI)。结果表明,与 K-PHI 相比,所获得的 H-PHI 在可见光照射下,在有铂纳米颗粒存在的情况下,在乙醇制氢反应中表现出极高的光催化活性。这种提高可归因于光生电荷转移到光催化剂表面的效率提高,而氧化还原过程就发生在光催化剂表面。对影响该系统活性的各种因素进行了评估。值得注意的是,研究发现酸引入系统的条件会显著影响 H-PHI 表面铂(助催化剂金属)沉积的大小,从而提高光催化系统产生分子氢的稳定性。研究证实,在光催化剂表面没有额外成分阻挡空气进入的情况下,该系统可以在空气存在的情况下高效运行。在最佳条件下,410 纳米波长下产生分子氢的表观量子产率约为 73%,这是迄今为止氮化碳材料的最高值。添加酸不仅能提高系统还原部分的活性,还能以高选择性从乙醇-1,1-二乙氧基乙烷(缩醛)中生成增值产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


