Partial closure of the γ-tubulin ring complex by CDK5RAP2 activates microtubule nucleation
IF 12.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Microtubule nucleation is templated by the γ-tubulin ring complex (γ-TuRC), but its structure deviates from the geometry of α-/β-tubulin in the microtubule, explaining the complex’s poor nucleating activity. Several proteins may activate the γ-TuRC, but the mechanisms underlying activation are not known. Here, we determined the structure of the porcine γ-TuRC purified using CDK5RAP2’s centrosomin motif 1 (CM1). We identified an unexpected conformation of the γ-TuRC bound to multiple protein modules containing MZT2, GCP2, and CDK5RAP2, resulting in a long-range constriction of the γ-tubulin ring that brings it in closer agreement with the 13-protofilament microtubule. Additional CDK5RAP2 promoted γ-TuRC decoration and stimulated the microtubule-nucleating activities of the porcine γ-TuRC and a reconstituted, CM1-free human complex in single-molecule assays. Our results provide a structural mechanism for the control of microtubule nucleation by CM1 proteins and identify conformational transitions in the γ-TuRC that prime it for microtubule nucleation.
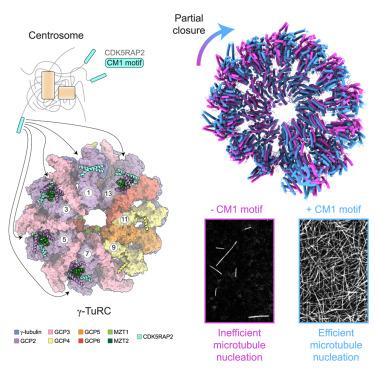
CDK5RAP2 对γ-微管蛋白环复合物的部分封闭可激活微管成核作用
微管成核是由γ-微管蛋白环复合物(γ-TuRC)模板化的,但其结构偏离了微管中α-/β-微管蛋白的几何结构,这就是该复合物成核活性差的原因。有几种蛋白质可能会激活γ-TuRC,但其激活机制尚不清楚。在这里,我们测定了利用 CDK5RAP2 的中心蛋白基序 1(CM1)纯化的猪γ-TuRC 的结构。我们发现了γ-TuRC与包含MZT2、GCP2和CDK5RAP2的多个蛋白质模块结合后的一种意想不到的构象,这种构象导致γ-tubulin环的长程收缩,使其更接近13原丝微管。在单分子实验中,额外的 CDK5RAP2 促进了 γ-TuRC 的装饰,并刺激了猪 γ-TuRC 和重组的、不含 CM1 的人类复合物的微管成核活性。我们的研究结果为 CM1 蛋白控制微管成核提供了一种结构机制,并确定了γ-TuRC 中使微管成核成为可能的构象转变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


