High-throughput screening of axially bonded dual atom catalysts for enhanced electrocatalytic reactions: The effect of van der Waals interaction
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
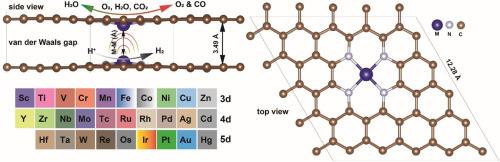
Single and dual-atom catalysts (SACs and DACs) on single-layer graphene are widely investigated for a wide range of electrochemical reactions. However, the effect of van der Waals interactions on the activity of these catalysts has not been investigated through systematic high throughput screening. Here we introduce the concept of van der Waals interactions through a double-layer DAC structure which has axial d orbital modification towards enhanced CO2 reduction reaction (CO2RR), hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and oxygen evolution reaction (OER). We applied density functional theory (DFT) to screen 3d, 4d, and 5d transition metals supported by double-layer nitrogen-doped graphene, denoted as M2N8. We sought catalysts with high thermodynamic and electrochemical stabilities along with low overpotentials for CO2RR, ORR, OER, or HER. We find that HER can take place inside the van der Waals gap of V2N8 and Co2N8 leading to overpotentials of 0.10 and 0.16 V. Moreover, ORR and OER can take place on the surface of Fe2N8 and Ir2N8, respectively, leading to overpotentials of 0.39 and 0.37 V. DFT predicts a CO2RR overpotential of 0.85 V towards CO on the surface of Co2N8 along with the HER overpotential of 0.16 V inside the van der Waals gap of Co2N8 towards the production of syngas (CO+H2). This paper provides fundamental insights into the design of advanced multi-layer catalysts by applying the concept of van der Waals interactions for electrochemistry at room temperature.
高通量筛选轴键双原子催化剂以增强电催化反应:范德华相互作用的影响
单层石墨烯上的单原子和双原子催化剂(SACs 和 DACs)已被广泛研究用于多种电化学反应。然而,范德华相互作用对这些催化剂活性的影响尚未通过系统的高通量筛选得到研究。在此,我们通过双层 DAC 结构引入了范德华相互作用的概念,该结构具有轴向 d 轨道修饰,可增强二氧化碳还原反应(CO2RR)、氢进化反应(HER)、氧还原反应(ORR)和氧进化反应(OER)。我们应用密度泛函理论(DFT)筛选了由双层掺氮石墨烯(记为 M2N8)支撑的 3d、4d 和 5d 过渡金属。我们寻找的催化剂具有较高的热力学和电化学稳定性,以及较低的 CO2RR、ORR、OER 或 HER 过电位。我们发现,HER 可在 V2N8 和 Co2N8 的范德华间隙内发生,过电势分别为 0.10 和 0.16 V。此外,ORR 和 OER 可分别在 Fe2N8 和 Ir2N8 表面发生,导致 0.39 V 和 0.37 V 的过电位。根据 DFT 预测,在 Co2N8 表面对 CO 的 CO2RR 过电位为 0.85 V,而在 Co2N8 的范德华间隙内对合成气(CO+H2)的 HER 过电位为 0.16 V。本文通过在室温电化学中应用范德华相互作用的概念,为设计先进的多层催化剂提供了基本见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


