Tunability in electronic and optical properties of GaS/PbS vdW heterostructure
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A promising novel class of heterostructures has recently emerged, combining a semiconducting GaS monolayer with other 2D materials for energy-related applications. In this study, we considered the layered PbS to form the van der Waals heterostructure with GaS and investigated its properties using density functional theory. The GaS/PbS heterostructure exhibits a type-II heterostructure with an indirect bandgap of 1.65 eV, displaying enhanced light absorption across the visible spectrum. Moreover, the heterostructure's energy band gap shows tunability with an applied transverse electric field attributed to the spontaneous electric polarization within the lattice. Subsequently, it contributes to increased optical absorbance and light harvesting efficiency under ±0.2 V/Å electric field. The applied electric field also offers tunable band alignments (transition type-II and type-I), making it a potential candidate for solar cells that can optimize their efficiency based on varying light conditions.
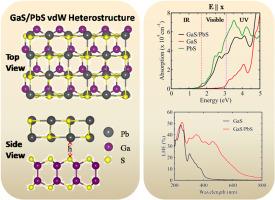
GaS/PbS vdW 异质结构的电子和光学特性的可调谐性
最近出现了一类前景广阔的新型异质结构,它将半导体 GaS 单层与其他二维材料结合起来,用于能源相关应用。在本研究中,我们考虑用层状 PbS 与 GaS 形成范德华异质结构,并利用密度泛函理论研究了其性质。GaS/PbS 异质结构是一种 II 型异质结构,其间接带隙为 1.65 eV,在可见光谱范围内具有增强的光吸收能力。此外,异质结构的能带隙在施加横向电场时显示出可调谐性,这归因于晶格内的自发电极化。因此,在 ±0.2 V/Å 电场条件下,它有助于提高光吸收率和光收集效率。外加电场还提供了可调的带排列(II 型和 I 型转变),使其成为太阳能电池的潜在候选材料,可根据不同的光照条件优化其效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


