Deep blue high-efficiency solution-processed triplet-triplet annihilation organic light-emitting diodes using bis(8-carbazol-N-yl)fluorene- and benzonitrile-modified anthracene/chrysene fluorescent emitters
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-09-19
DOI:10.1016/j.jphotochem.2024.116046
引用次数: 0
Abstract
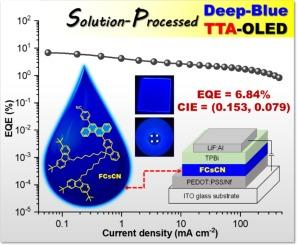
Triplet-triplet annihilation (TTA) emitters can effectively utilize non-radiative triplet excitons through the interaction of low triplet energy excitons to produce high energy singlet excitons, but they are mostly restricted by their multilayered device structure fabricated using layer-by-layer thermal vacuum evaporation. It is a great challenge to develop, for the first time, efficient solution-processed non-doped TTA organic light-emitting diodes (OLEDs). In this study, two solution-processable blue emissive TTA molecules (FAnCN and FCsCN) bearing (anthracen-9-yl)benzonitrile (AnCN) and (chrysen-6-yl)benzonitrile (CsCN) as TTA emissive cores modified with 9,9′-bis(8-(carbazole-N-yl)octyl)fluorene (F) are designed and synthesized, respectively. The experimental and theoretical studies reveal that both molecules exhibit deep blue emissions, amorphous morphology with good thermal stability, high-quality solution-cast thin films, decent hole mobility, high-lying HOMO levels (∼-5.45 eV), and suitable lowest singlet (S1)/triplet (T1) excited states (2T1 > S1) for TTA process. FAnCN and FCsCN are successfully employed as solution-processed non-doped emissive layers (EML) in simple structured TTA OLEDs. These devices show intense blue emissions, low turn-on voltages (∼3.6 V), excellent electroluminescent (EL) performances (EQEmax = 5.47–6.84 % and LEmax = 5.66–5.83 cd/A), and TTA characteristics. Especially, FCsCN-based TTA OLED emits deep blue EL emission peaked at 435 nm with a high EQEmax of 6.84 %. This work not only presents a new strategic design for the preparation of solution-processable TTA emitter, but also further ratifies that the TTA mechanism can also be applicable in solution-processed OLEDs.
使用双(8-咔唑-N-基)芴和苯甲腈改性蒽/菊烯荧光发射器的深蓝色高效溶液处理三重-三重湮灭有机发光二极管
三重三重湮灭(TTA)发光体可通过低三重能激子的相互作用产生高能量单重激子,从而有效利用非辐射三重激子,但它们大多受限于采用逐层热真空蒸发法制造的多层器件结构。如何首次开发出高效的溶液工艺非掺杂 TTA 有机发光二极管(OLED)是一项巨大的挑战。本研究设计并合成了两种可溶液加工的蓝色发射型 TTA 分子(FAnCN 和 FCsCN),它们分别以(蒽-9-基)苯甲腈(AnCN)和(克利森-6-基)苯甲腈(CsCN)为 TTA 发射核,并用 9,9′-双(8-(咔唑-N-基)辛基)芴(F)修饰。实验和理论研究表明,这两种分子都具有深蓝色发射光谱、热稳定性良好的无定形形貌、高质量的溶液浇铸薄膜、良好的空穴迁移率、高位 HOMO 水平(∼-5.45 eV)以及适合 TTA 过程的最低单态(S1)/三态(T1)激发态(2T1 > S1)。FAnCN 和 FCsCN 被成功地用作简单结构 TTA 有机发光二极管中的溶液加工非掺杂发射层(EML)。这些器件显示出强烈的蓝色发射、较低的开启电压(∼3.6 V)、优异的电致发光(EL)性能(EQEmax = 5.47-6.84 % 和 LEmax = 5.66-5.83 cd/A)以及 TTA 特性。特别是,基于 FCsCN 的 TTA OLED 在 435 纳米波长处发出深蓝色 EL 发射,EQEmax 高达 6.84 %。这项工作不仅为制备可溶液加工的 TTA 发射器提供了一种新的战略设计,而且进一步证实了 TTA 机制也可应用于溶液加工的有机发光二极管。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


