Humic substances and inorganic ions synergistically precipitate at the gas–liquid interface in a simulation experiment of direct-contact heat and mass transfer processes
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Direct-contact heat and mass transfer processes are widely employed in saline wastewater treatment. However, these processes are accompanied by serious ultrafine particle emission, a phenomenon associated with the solute’s transition across the gas–liquid interface. A thorough comprehension of the solute’s behavior at this gas–liquid interface is vital for clarifying the crossing process and guiding particle control strategies. Here, solutions containing saturated inorganic ions and humic substances were evaporated to analyze their behaviors at the gas–liquid interface in an experimental simulation of direct-contact heat and mass transfer processes. It was found that these substances co-precipitated at this interface. Humic substances can bind with inorganic ions via chemical bonds, monovalent ions through cation-π interactions, and divalent ions through electrostatic and chelation interactions. Moreover, humic substances can act as nucleation sites for precipitating inorganic crystals. These distinctive binding mechanisms caused a synergistic relationship between humic substances and inorganic ions during precipitation. Amidst precipitation, humic substances with high humification degrees and large molecular weights were predominantly enriched. Inorganic ions, comprising over 93% of the total precipitate mass, constituted the principal constituents. Among these, Na+, with an enrichment factor of 2.10, precipitated more readily at the gas–liquid interface compared to divalent ions. These conclusions concerning the binding mechanisms of humic substances and inorganic ions, along with their precipitation characteristics, were validated in the submerged combustion evaporation process of membrane-concentrated leachate.
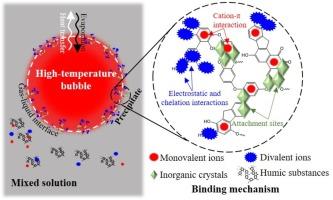
在直接接触传热和传质过程的模拟实验中,腐殖质和无机离子在气液界面协同沉淀
直接接触传热和传质过程被广泛应用于含盐废水处理中。然而,这些过程伴随着严重的超细颗粒排放,这种现象与溶质跨越气液界面的转变有关。透彻理解溶质在气液界面上的行为对于澄清穿越过程和指导颗粒控制策略至关重要。在此,我们蒸发了含有饱和无机离子和腐殖质的溶液,在直接接触传热和传质过程的实验模拟中分析了它们在气液界面上的行为。结果发现,这些物质在该界面上共沉淀。腐殖质可通过化学键与无机离子结合,通过阳离子-π相互作用与一价离子结合,通过静电和螯合作用与二价离子结合。此外,腐殖质还可以作为无机晶体沉淀的成核场所。这些独特的结合机制使腐殖质与无机离子在沉淀过程中产生了协同作用。在沉淀过程中,主要富集的是腐殖化程度高、分子量大的腐殖质。无机离子是主要成分,占沉淀物总质量的 93% 以上。其中,富集系数为 2.10 的 Na+ 比二价离子更容易在气液界面沉淀。这些关于腐殖质和无机离子的结合机制及其沉淀特性的结论在膜浓缩浸出液的浸没燃烧蒸发过程中得到了验证。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


