Theoretical study on borophene as metal-free catalyst for selective conversion of NO
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
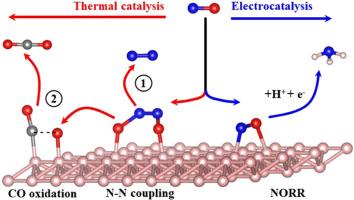
The electrocatalytic reduction of NO (NORR) and selective catalytic reduction of NO by CO (CO-SCR) are the two most attractive approaches for selective conversion of NO. Herein, a bifunctional metal-free catalyst 2-Pmmn borophene is reported that is effective for both NORR and CO-SCR. NO can form NH3 and N2 through NORR and CO-SCR respectively. The results show that NO chemically adsorbed on the surface of borophene through the N![]() O terminal can be electrocatalytically reduced to NH3. The optimal reaction path for NO to generate NH3 is through the protonation process of *NHO instead of *NOH. The rate-determining step is the hydrogenation of *NH2O to *NH2OH, and the free energy increases by 0.41 eV. At the same time, NO can also react with CO on the surface of borophene to form N2 and CO2. First, NO can form chemically adsorbed ONNO intermediate through N
O terminal can be electrocatalytically reduced to NH3. The optimal reaction path for NO to generate NH3 is through the protonation process of *NHO instead of *NOH. The rate-determining step is the hydrogenation of *NH2O to *NH2OH, and the free energy increases by 0.41 eV. At the same time, NO can also react with CO on the surface of borophene to form N2 and CO2. First, NO can form chemically adsorbed ONNO intermediate through N![]() N coupling, then ONNO can be denitrified to form N2 and residual oxygen, and finally residual oxygen and CO can generate CO2 through the LH mechanism. The rate-determining step of the reaction is the N
N coupling, then ONNO can be denitrified to form N2 and residual oxygen, and finally residual oxygen and CO can generate CO2 through the LH mechanism. The rate-determining step of the reaction is the N![]() N coupling process of NO, and activation energy barrier is 1.27 eV. The present work provides theoretical insights for the effective conversion of NO.
N coupling process of NO, and activation energy barrier is 1.27 eV. The present work provides theoretical insights for the effective conversion of NO.
硼菲作为无金属催化剂选择性转化氮氧化物的理论研究
电催化还原一氧化氮(NORR)和一氧化碳选择性催化还原一氧化氮(CO-SCR)是选择性转化一氧化氮的两种最有吸引力的方法。本文报告了一种双功能无金属催化剂 2-Pmmn 硼吩,它对 NORR 和 CO-SCR 均有效。通过 NORR 和 CO-SCR,NO 可分别生成 NH3 和 N2。研究结果表明,通过 NO 端化学吸附在硼吩表面的 NO 可以通过电催化还原成 NH3。NO 生成 NH3 的最佳反应路径是通过 *NHO 而不是 *NOH 的质子化过程。决定速率的步骤是 *NH2O 加氢为 *NH2OH,自由能增加了 0.41 eV。与此同时,NO 还能在硼吩表面与 CO 反应生成 N2 和 CO2。首先,NO 可以通过 NN 偶联形成化学吸附的 ONNO 中间体,然后 ONNO 被反硝化形成 N2 和残氧,最后残氧和 CO 通过 LH 机制生成 CO2。该反应的速率决定步骤是 NO 的 NNN 偶联过程,活化能垒为 1.27 eV。本研究为 NO 的有效转化提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


