CO2 hydrogenation to light olefins over highly active and selective Ga-Zr/SAPO-34 bifunctional catalyst
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
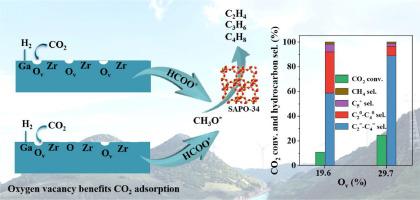
The production of light olefins from the hydrogenation of CO2 is an efficient way to utilize CO2, where the surface oxygen vacancy in metal oxide plays an important role in CO2 adsorption and activation. Here, the Ga-Zr metal oxides were prepared by hydrolysis of urea at different temperatures and combined with SAPO-34 to prepare the bifunctional catalyst for CO2 hydrogenation to light olefins. The surface oxygen vacancy content of Ga-Zr oxide increases with increasing urea hydrolysis temperature, and a high CO2 conversion of 26.4% and C2=–C4= hydrocarbon selectivity of 87.2% were obtained by a well-matched amount of desorbed CO2 and H2. Using the CO2 and H2/HCOOH/CH3OH as probe molecules, the in-situ DRIFT spectra reveal that the CO2 could be activated on surface oxygen vacancy and converted to CO3* and HCO3* species, which were further hydrogenated to HCOO* and CH3O* species. While the by-product CO mainly originates from the decomposition of HCOO* and the presence of SAPO-34 converts CH3O* to C2=–C4=. The current study illustrates that boosting the surface oxygen vacancy in defected surfaces of metal oxide and providing a matching H2 dissociation ability is the key to improve the performance of CO2 hydrogenation to light olefins.
在高活性、高选择性 Ga-Zr/SAPO-34 双功能催化剂上将二氧化碳加氢制取轻烯烃
二氧化碳加氢制取轻烯烃是利用二氧化碳的一种有效方法,其中金属氧化物表面的氧空位在二氧化碳的吸附和活化中起着重要作用。本文通过在不同温度下水解尿素制备了 Ga-Zr 金属氧化物,并与 SAPO-34 结合制备了用于 CO2 加氢制取轻质烯烃的双功能催化剂。Ga-Zr 氧化物的表面氧空位含量随尿素水解温度的升高而增加,通过良好匹配的 CO2 和 H2 解吸量,获得了 26.4% 的高 CO2 转化率和 87.2% 的 C2=-C4= 碳氢化合物选择性。以 CO2 和 H2/HCOOH/CH3OH 为探针分子,原位 DRIFT 图谱显示,CO2 可在表面氧空位上被活化并转化为 CO3* 和 HCO3* 物种,然后进一步氢化为 HCOO* 和 CH3O* 物种。本研究表明,提高金属氧化物缺陷表面的表面氧空位并提供与之相匹配的 H2 解离能力是改善 CO2 加氢制轻烯烃性能的关键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


