Application of computational techniques on non-covalent interactions, H-bond nature of monomeric and dimeric form of crystal structures, and topological insights of glycine glutaric acid
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This study utilizes DFT to investigate and optimize the structure of Glycine Glutaric acid (GGA) crystal in both monomer and dimer forms, assessing its electronic and optical properties. Relaxed PES scanning identified potential conformers within the COOH and NH2 functional groups. FT-IR spectrum confirmed these groups and simulated spectra were correlated with the experimental data. The stable monomer was selected for detailed analysis of electronic charge transfer using MEP, FMOs, and UV–visible absorbance spectra. Non-covalent interactions, primarily O–H⋯O and N–H⋯O hydrogen bonds, were explored using optimized structures. Solvent effects, analyzed via the IEFPCM method, revealed heightened reactivity in the aqueous phase. Topological studies (AIM, LOL, ELF, and RDG) and Hirshfeld surface analysis were applied to understand inter and intramolecular contacts, with crystal packing dominated by O⋯H/H⋯O interactions contributing to 63.4 % efficiency. As per DFT prediction, the GGA exhibits strong NLO potential due to significantly higher polarizability and hyperpolarizability ( 1.3618 × 10−30 e.s.u.) indicating promising nonlinear optical properties.
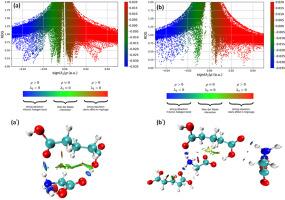
应用计算技术研究甘氨酸戊二酸的非共价相互作用、晶体结构单体和二聚体的氢键性质以及拓扑学原理
本研究利用 DFT 研究和优化了单体和二聚体形式的甘氨酸-戊二酸(GGA)晶体结构,评估了其电子和光学特性。松弛 PES 扫描确定了 COOH 和 NH2 官能团内的潜在构象。傅立叶变换红外光谱证实了这些基团,模拟光谱与实验数据相关联。我们选择了稳定的单体,利用 MEP、FMO 和紫外-可见吸收光谱对电子电荷转移进行了详细分析。利用优化结构探索了非共价相互作用,主要是 O-H⋯O 和 N-H⋯O 氢键。通过 IEFPCM 方法分析的溶剂效应显示,水相中的反应活性有所提高。拓扑研究(AIM、LOL、ELF 和 RDG)和 Hirshfeld 表面分析被用来了解分子间和分子内的接触,O⋯H/H⋯O 相互作用主导了晶体的堆积,从而提高了 63.4% 的效率。根据 DFT 预测,由于极化率和超极化率显著提高(⟨β⟩= 1.3618 × 10-30 e.s.u.),GGA 具有很强的非线性光学潜力,显示出良好的非线性光学特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


