Application of Response Surface Methodology for concentration of fluorosilicic acid by extraction technique with benzyl alcohol as extractant
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
As a kind of alternative of natural fluorinated minerals and raw material for producing anhydrous hydrogen fluoride, fluorosilicic acid has attracted more and more attention. In order to raise its utilization rate, dilute fluorosilicic acid solution coming from chemical enterprises needs to be concentrated. The extraction technology with benzyl alcohol as extraction agent was used to concentrating fluorosilicic acid aqueous solution. In order to determine the optimizing operation conditions of the extracting process, single - factor experiments were carried out to confirm the significant affecting factors and their operating range. Then the Response Surface Methodology (RSM) was conducted to accomplish regression of a nonlinear quadratic polynomial model to the experimental data and statistical analysis. Based on these studies, the optimal technical parameters of extracting process are acquired by optimization of the model using the Design-Expert 13 software. The statistical analysis for the fitting response surface quadratic equation model indicated that the model was reliable and accurate with very low p-values (<0.0001), the predicted values with the model equation had remarkable consistency in experimental data. By a great number of optimizations carried out with the Design Expert 13 software based on the fitting model equation, the optimal extractive processing conditions for concentrating fluorosilicic acid aqueous solution were obtained: the phase ratio 7, the extraction time 120.11 min and the extraction temperature 39.70 °C. The optimal concentration of fluorosilicic acid is 26.68 %. In order to increase the concentration of fluorosilicic acid, three-stage cross-current extraction for dilute fluorosilicic acid solution was carried out at the optimizing extracting conditions, 17.43 wt% fluorosilicic acid solution can be concentrated up to 37.46 wt%. The extractant-benzyl alcohol can be recovered with vacuum distillation and recovery rate reached up to 98 %.
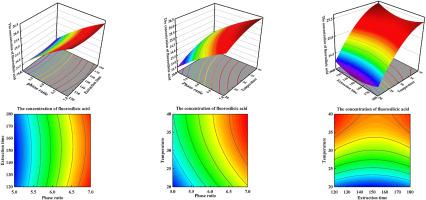
响应面法在以苯甲醇为萃取剂的萃取技术浓缩氟硅酸中的应用
氟硅酸作为一种天然含氟矿物的替代品和生产无水氟化氢的原料,越来越受到人们的关注。为了提高氟硅酸的利用率,需要对来自化工企业的稀氟硅酸溶液进行浓缩。以苯甲醇为萃取剂的萃取技术被用于浓缩氟硅酸水溶液。为了确定萃取工艺的优化操作条件,进行了单因素实验,以确定重要的影响因素及其操作范围。然后采用响应面法(RSM)对实验数据进行非线性二次多项式模型回归和统计分析。在这些研究的基础上,通过使用 Design-Expert 13 软件对模型进行优化,获得了提取工艺的最佳技术参数。拟合响应面二次方程模型的统计分析结果表明,该模型可靠、准确,P 值非常低(<0.0001),模型方程的预测值与实验数据具有显著的一致性。根据拟合的模型方程,利用 Design Expert 13 软件进行了大量的优化,得到了浓缩氟硅酸水溶液的最佳萃取处理条件:相比 7,萃取时间 120.11 min,萃取温度 39.70 °C。氟硅酸的最佳浓度为 26.68%。为了提高氟硅酸的浓度,在优化萃取条件下对稀氟硅酸溶液进行了三级逆流萃取,17.43 wt%的氟硅酸溶液可以浓缩到 37.46 wt%。萃取剂-苄醇可通过真空蒸馏回收,回收率高达 98%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


