Synthesis, characterization, anticancer activity, molecular docking and DFT calculation of 3-acetylcoumarin thiosemicarbazones and Schiff’s bases
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
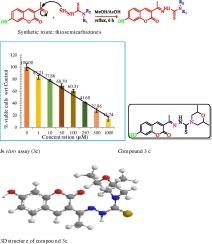
The new thiosemicarbazones (3a-d) of 3-acetylumbelliferone and Schiff’s bases (4a-b) of 3-acetylcoumarin were synthesized, characterized by elemental analysis, UV–Vis, FT-IR, NMR, and ESI-HR MS, and evaluated for their anticancer potency against the human breast cancer (MCF-7) cell line. All the tested compounds exhibited good to moderate anticancer efficacy in a dose-dependent manner. The synthesized compounds exhibited potent preferential inhibition effects against the MCF-7 cell line, with IC50 range of 147.8–505.1 µg/mL. The most potent compound, (E)-N-(1-(7-hydroxy-2-oxo-2H-chromen-3-yl) ethylidine)-2,6-dimethylmorpholine-4-carbothiohydrazide (3c), showed significant cytotoxicity with an IC50 value of 147.8 µg/mL against the tested cell line. It showed a higher proportion of cells in G1 (37.21 %) and S (26.86 %) phases and a lower proportion of cells in G2 phase (26.37 %), with respect to control. Molecular docking of all the synthesized compounds with target proteins VEGFR2 and EGFR demonstrated their significant binding affinity and interactions with key residues compared to the reference drug (erlotinib). DFT-based analysis showed that all the compounds have effective reactivity due to the low band gap energy of HOMO and LUMO relative to the reference drug, erlotinib (4.26 eV). The reactivity-related quantum mechanical parameters η, S, χ, μ, and ω indicated that these compounds have a high efficacy towards the target enzymes VEGFR2 and EGFR. All of the synthesized compounds had drug-like action, according to ADMET predictions.
3-acetylcoumarin thiosemicarbazones 和 Schiff's 碱的合成、表征、抗癌活性、分子对接和 DFT 计算
合成了 3-acetylumbelliferone 的新硫代氨基甲酸(3a-d)和 3-acetylcoumarin 的希夫碱(4a-b),并通过元素分析、紫外-可见光谱、傅立叶变换红外光谱、核磁共振和 ESI-HR MS 对其进行了表征,还评估了它们对人类乳腺癌(MCF-7)细胞系的抗癌效力。所有受试化合物都表现出良好至中等程度的抗癌效力,其抗癌效力与剂量有关。合成的化合物对 MCF-7 细胞系具有强效的优先抑制作用,IC50 范围为 147.8-505.1 µg/mL。最有效的化合物是(E)-N-(1-(7-羟基-2-氧代-2H-苯并吡喃-3-基)乙脒)-2,6-二甲基吗啉-4-硫代酰肼(3c),它对受试细胞系具有显著的细胞毒性,IC50 值为 147.8 µg/mL。与对照组相比,处于 G1 期(37.21 %)和 S 期(26.86 %)的细胞比例较高,而处于 G2 期(26.37 %)的细胞比例较低。所有合成化合物与靶蛋白血管内皮生长因子受体2(VEGFR2)和表皮生长因子受体(EGFR)的分子对接结果表明,与参比药物(厄洛替尼)相比,它们具有显著的结合亲和力以及与关键残基的相互作用。基于 DFT 的分析表明,与参考药物厄洛替尼(4.26 eV)相比,所有化合物的 HOMO 和 LUMO 带隙能较低,因此具有有效的反应活性。与反应活性相关的量子力学参数η、S、χ、μ和ω表明,这些化合物对靶酶VEGFR2和表皮生长因子受体具有很高的药效。根据 ADMET 预测,所有合成的化合物都具有类似药物的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


