Dimerization of perfluoropropyl vinyl ether during the pyrolysis of hexafluoropropylene oxide dimer
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
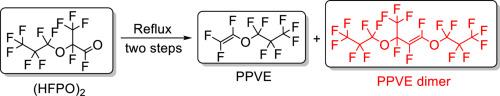
Dimerization of perfluoropropyl vinyl ether (PPVE) generally reduces its yield and selectivity during the pyrolysis of hexafluoropropylene oxide dimer ((HFPO)2). However, the mechanism of PPVE dimerization is not well understood. In this paper, the PPVE dimer was obtained during the pyrolysis of (HFPO)2. Subsequently, the chemical structure of PPVE dimer was further determined by gas chromatography-mass spectrometry (GC–MS) and nuclear magnetic resonance (NMR). The results showed that the concentration of PPVE dimer rises in proportion to the extended reflux time of PPVE in the reaction system. Based on the experimental phenomenon, a possible generation mechanism of PPVE dimer was then proposed, and the possibility of the generation pathway was further verified in combination with density functional theory (DFT). In addition, to effectively reduce the production of PPVE dimer, crown ethers and quaternary ammonium salts were added to the reaction system as phase transfer catalysts. Among them, the phase transfer catalyst (15-crown-5) was more effective and reduced the PPVE dimer content from 1.34 % to 0.31 %. This work provides an idea to inhibit the dimerization of PPVE and increase the yield and selectivity of PPVE.
全氟丙基乙烯基醚在六氟环氧丙烷二聚物热解过程中的二聚反应
在热解六氟环氧丙烷二聚物((HFPO)2)的过程中,全氟丙基乙烯基醚(PPVE)的二聚化通常会降低其产率和选择性。然而,人们对 PPVE 二聚化的机理并不十分清楚。本文通过热解 (HFPO)2 获得了 PPVE 二聚物。随后,通过气相色谱-质谱法(GC-MS)和核磁共振法(NMR)进一步确定了 PPVE 二聚体的化学结构。结果表明,随着 PPVE 在反应体系中回流时间的延长,PPVE 二聚体的浓度呈正比例上升。根据实验现象,提出了 PPVE 二聚体的可能生成机制,并结合密度泛函理论(DFT)进一步验证了生成途径的可能性。此外,为了有效减少 PPVE 二聚物的生成,在反应体系中加入了冠醚和季铵盐作为相转移催化剂。其中,相转移催化剂(15-冠醚-5)的效果更好,可将 PPVE 二聚物的含量从 1.34% 降至 0.31%。这项工作为抑制 PPVE 的二聚化、提高 PPVE 的产率和选择性提供了思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


