Selective separation of polyphenols with a novel azo porous organic polymer: Ultrahigh adsorption capacity and superfast adsorption rate
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
For the first time, a novel porous organic polymer (POP), namely Cin-POP, was constructed under normal temperature and pressure using less toxic basic fuchsin and natural cinnamic acid. Cin-POP was synthesized through the diazo-coupling reaction between the diazonium salts of basic fuchsin and cinnamic acid. The FT-IR spectra and XPS analysis prove the successful construction of the Cin-POP, and the BET analysis demonstrates the high-surface-area (166.42–202.56 m2 g−1) and suitable pore sizes (11.00–18.63 nm). Owing to the advantages of porous structures, abundant aromatic groups, good thermal stability, and good dispersity, Cin-POP exhibits remarkable adsorption rates and capacities towards polyphenols, including procyanidine and rutin. Especially for procyanidine, the extraction efficiency can arrive at nearly 98.1 % after 2.5 min adsorption, and the pseudo-second-order rate constant (k2) of Cin-POP is 0.0257 g mg−1 min−1. The maximum adsorption capacities of Cin-POP towards procyanidine and rutin are 2500.00 mg g−1 and 1814.83 mg g−1, outpacing all reported adsorbents. In addition, Cin-POP can selectively adsorb polyphenol among the mixtures of polyphenols and alkaloids. More importantly, Cin-POP can be used for polyphenols separation from complex cinnamon extract. All the above advantages make Cin-POP comparable porous adsorbent for polyphenol separation.
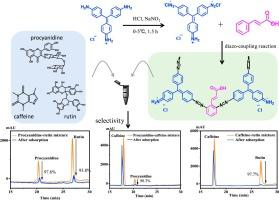
利用新型偶氮多孔有机聚合物选择性分离多酚:超高吸附容量和超快吸附速率
利用毒性较低的碱性紫苏和天然肉桂酸,首次在常温常压下构建了一种新型多孔有机聚合物(POP),即 Cin-POP。Cin-POP 是通过碱性品红的重氮盐与肉桂酸的重氮偶联反应合成的。傅立叶变换红外光谱和 XPS 分析证明了 Cin-POP 的成功构建,BET 分析表明了其高比表面积(166.42-202.56 m2 g-1)和合适的孔径(11.00-18.63 nm)。由于具有多孔结构、丰富的芳香基团、良好的热稳定性和分散性等优点,Cin-POP 对包括原花青素和芦丁在内的多酚具有显著的吸附率和吸附容量。特别是对原花青素,吸附 2.5 分钟后萃取效率可达近 98.1%,Cin-POP 的假二阶速率常数(k2)为 0.0257 g mg-1 min-1。Cin-POP 对原花青素和芦丁的最大吸附容量分别为 2500.00 mg g-1 和 1814.83 mg g-1,超过了所有已报道的吸附剂。此外,Cin-POP 还能选择性地吸附多酚和生物碱混合物中的多酚。更重要的是,Cin-POP 可用于从复杂的肉桂提取物中分离多酚。所有这些优点使 Cin-POP 成为可用于多酚分离的多孔吸附剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


