Gabapentin: An impurity profiling approach with hydrogen peroxide induced thermal oxidation in autoclave
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Predicting unconventional pathways in long term stability studies can be very challenging, especially in solid state degradation. This is of vital importance for enhancement in the level of degradation and to further enable the isolation of unknown degradation products. Predicting the mechanism or pathway also help in designing control strategies to minimise the potential chances of impurity formation. In this context, the lagtime of most oxidative degradation mechanisms is extremely important, which allows enough requisite of free radicals etc., one of the unconventional pathways. This is difficult to replicate in degradation studies involving usual solution form. In this investigation, we demonstrate that employing autoclave degradation with hydrogen peroxide conditions provide an enhanced level of an unknown degradation product, which was found to be futile in traditional forced degradation studies. The conditions used probably bypass the lag time. This impurity has been isolated using preparative liquid chromatography (Prep-HPLC) method and spectroscopic techniques like high-resolution mass spectrometry (HRMS) and nuclear magnetic resonance NMR (1H, 13C, DEPT-135, NOESY, APT) for elucidation of the molecular structure. The impurity was identified as a dimer of gabapentin impurity-A (impurity (2,2′-diaza[l,2′-bispiro[4.5]decane]-3,3′-dione) and named it as impurity-U. A plausible mechanism for the formation of isolated impurity is proposed. HPLC methods have been developed and reported for the determination of gabapentin and its related substances using UV detection.
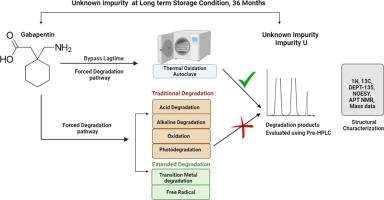
加巴喷丁利用高压釜中过氧化氢诱导热氧化的杂质分析方法
在长期稳定性研究中预测非常规途径是一项非常具有挑战性的工作,尤其是在固态降解方面。这对于提高降解水平和进一步分离未知降解产物至关重要。预测降解机理或途径还有助于设计控制策略,最大限度地减少杂质形成的潜在机会。在这种情况下,大多数氧化降解机制的滞后性极为重要,这使得自由基等非常规途径有足够的必要条件。这在涉及普通溶液形式的降解研究中很难复制。在这项研究中,我们证明了在过氧化氢条件下使用高压釜降解可以提高未知降解产物的水平,而这在传统的强制降解研究中是徒劳的。所使用的条件可能绕过了滞后时间。我们采用制备液相色谱法(Prep-HPLC)和高分辨率质谱法(HRMS)、核磁共振核磁共振法(1H、13C、DEPT-135、NOESY、APT)等光谱技术分离出了这种杂质,以阐明其分子结构。经鉴定,该杂质是加巴喷丁杂质-A(杂质(2,2′-二氮杂[l,2′-双螺[4.5]癸烷]-3,3′-二酮)的二聚体,并将其命名为杂质-U。提出了分离出的杂质的合理形成机制。开发并报告了利用紫外检测法测定加巴喷丁及其相关物质的高效液相色谱法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


