Desulfonation-associated direct amide bond formation between N-sulfonyl-1,2,3-triazoles with carboxylic acids
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We have developed a highly N2-regioselective method for direct construction of amide bond from N-sulfonyl-1,2,3-triazoles and carboxylic acids in the presence of bases at 60 °C in air. The developed reaction provides the corresponding products with high yields (up to 90 %) and a broad substrate compatibility including aryl acids, heterocyclic acids, and alkyl acids. Mechanistic studies show that the reaction proceeds through a direct nucleophilic attack of N-sulfonyl-1,2,3-triazoles to carboxylate anions via a base- and water-involved synergistic desulfonation process. This work presents an unusual water-involved example for direct synthesis of amides utilizing readily available starting materials under mild conditions.
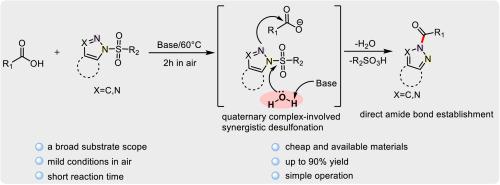
N-磺酰基-1,2,3-三唑与羧酸之间脱磺相关的直接酰胺键形成
我们开发了一种高度 N2 区域选择性的方法,用于在 60 °C 空气中,在碱存在的条件下,由 N-磺酰基-1,2,3-三唑和羧酸直接构建酰胺键。所开发的反应可提供相应的产物,且产率高(高达 90%),并具有广泛的底物兼容性,包括芳基酸、杂环酸和烷基酸。机理研究表明,该反应是通过 N-磺酰基-1,2,3-三唑对羧酸根阴离子的直接亲核攻击,经由碱和水参与的协同脱硫过程进行的。这项研究为在温和条件下利用容易获得的起始材料直接合成酰胺提供了一个不同寻常的水参与实例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


