Identification of natural curcumins as potential dual inhibitors of PTP1B and α-glucosidase through experimental and computational study
IF 1.2
4区 综合性期刊
Q3 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Curcuma longa is a rich source of curcumin (1) and its major analogs, demethoxycurcumin (2), and bisdemethoxycurcumin (3), among the Curcuma species. In this study, curcumins (1–3) were purified from C. longa, and their inhibitory effects against protein tyrosine phosphatase 1B (PTP1B) and α-glucosidase enzymes were assayed. These compounds potential inhibited PTP1B and α-glucosidase activities with IC50 values ranging from 37.8 to 72.6 μM, and 78.2–90.6 μM, respectively. In addition, density functional theory (DFT) accompanied by molecular docking (MD) were employed to analyze the ligand stability and the interaction of curcumins 1–3 with PTP1B and glucoside hydrolase proteins. The assay-based results and MD data obtained showed a high correlation, suggesting that the deterioration of enzyme activity caused by the distortion of the structural conformation of PTP1B and glucoside hydrolase may be related to the arrangement of amino acids in the protein structure. Our findings reveal the significant role of methoxylation in the variation of inhibitory effects of these curcumins against PTP1B and α-glucosidase. These in vitro and in silico activities of curcumins (1–3) against PTP1B and glucoside hydrolase have been examined and reported for the first time.
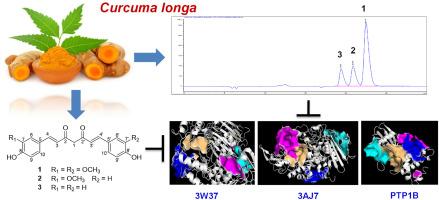
通过实验和计算研究发现天然姜黄素是 PTP1B 和 α-葡萄糖苷酶的潜在双重抑制剂
姜黄是姜黄属植物中姜黄素(1)及其主要类似物去甲氧基姜黄素(2)和双去甲氧基姜黄素(3)的丰富来源。本研究从姜黄中纯化了姜黄素(1-3),并检测了它们对蛋白酪氨酸磷酸酶 1B (PTP1B)和α-葡萄糖苷酶的抑制作用。这些化合物对 PTP1B 和 α-葡萄糖苷酶活性的潜在抑制作用的 IC50 值分别为 37.8 至 72.6 μM 和 78.2 至 90.6 μM。此外,还采用密度泛函理论(DFT)和分子对接理论(MD)分析了配体的稳定性以及姜黄素 1-3 与 PTP1B 和葡萄糖苷水解酶蛋白的相互作用。化验结果和 MD 数据显示出很高的相关性,表明 PTP1B 和葡糖苷水解酶结构构象的扭曲导致酶活性的降低可能与蛋白质结构中氨基酸的排列有关。我们的研究结果表明,甲氧基化在这些姜黄素对 PTP1B 和 α-葡萄糖苷酶抑制作用的变化中起着重要作用。这些姜黄素(1-3)对 PTP1B 和葡糖苷水解酶的体外和硅学活性是首次研究和报道。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kuwait Journal of Science
MULTIDISCIPLINARY SCIENCES-
CiteScore
1.60
自引率
28.60%
发文量
132
期刊介绍:
Kuwait Journal of Science (KJS) is indexed and abstracted by major publishing houses such as Chemical Abstract, Science Citation Index, Current contents, Mathematics Abstract, Micribiological Abstracts etc. KJS publishes peer-review articles in various fields of Science including Mathematics, Computer Science, Physics, Statistics, Biology, Chemistry and Earth & Environmental Sciences. In addition, it also aims to bring the results of scientific research carried out under a variety of intellectual traditions and organizations to the attention of specialized scholarly readership. As such, the publisher expects the submission of original manuscripts which contain analysis and solutions about important theoretical, empirical and normative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


