Linoleic Acid Promotes Mitochondrial Biogenesis and Alleviates Acute Lung Injury
Abstract
Introduction
Acute lung injury (ALI) is a critical and lethal medical condition. This syndrome is characterized by an imbalance in the body's oxidation stress and inflammation. Linoleic acid (LA), a polyunsaturated fatty acid, has been extensively studied for its potential health benefits, including anti-inflammatory and antioxidant activities. However, the therapeutic effects of LA on ALI remain unexplored.
Methods
Lipopolysaccharide (LPS), found in gram-negative bacteria's outer membrane, was intraperitoneally injected to induce ALI in mice. In vitro model was established by LPS stimulation of mouse lung epithelial 12 (MLE-12) cells.
Results
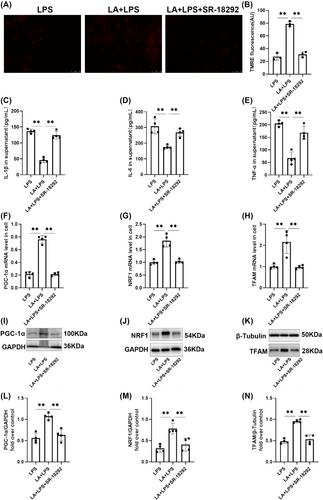
LA treatment demonstrated a significant amelioration in LPS-induced hypothermia, poor state, and pulmonary injury in mice. LA treatment resulted in a reduction in the concentration of bronchoalveolar lavage fluid (BALF) protein and an increase in myeloperoxidase (MPO) activity in LPS-induced mice. LA treatment reduced the generation of white blood cells. LA treatment reduced cell-free (cfDNA) release and promote adenosine triphosphate (ATP) production. LA increased the levels of superoxide dismutase (SOD) and glutathione (GSH) but decreased the production of malondialdehyde (MDA). LA treatment enhanced mitochondrial membrane potential. LA attenuated LPS-induced elevations of inflammatory cytokines in both mice and cells. Additionally, LA exerted its protective effect against LPS-induced damage through activation of the peroxisome proliferator-activated receptor γ coactivator l alpha (PGC-1α)/nuclear respiratory factor 1 (NRF1)/transcription factor A of the mitochondrion (TFAM) pathway.
Conclusion
LA may reduce inflammation and stimulate mitochondrial biogenesis in ALI mice and MLE-12 cells.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


