Strong Magnetic Exchange Interactions and Delocalized Mn–O States Enable High-Voltage Capacity in the Na-Ion Cathode P2–Na0.67[Mg0.28Mn0.72]O2
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The increased capacity offered by oxygen-redox active cathode materials for rechargeable lithium- and sodium-ion batteries (LIBs and NIBs, respectively) offers a pathway to the next generation of high-gravimetric-capacity cathodes for use in devices, transportation and on the grid. Many of these materials, however, are plagued with voltage fade, voltage hysteresis and O2 loss, the origins of which can be traced back to changes in their electronic and chemical structures on cycling. Developing a detailed understanding of these changes is critical to mitigating these cathodes’ poor performance. In this work, we present an analysis of the redox mechanism of P2–Na0.67[Mg0.28Mn0.72]O2, a layered NIB cathode whose high capacity has previously been attributed to trapped O2 molecules. We examine a variety of charge compensation scenarios, calculate their corresponding densities of states and spectroscopic properties, and systematically compare the results to experimental data: 25Mg and 17O nuclear magnetic resonance (NMR) spectroscopy, operando X-band and ex situ high-frequency electron paramagnetic resonance (EPR), ex situ magnetometry, and O and Mn K-edge X-ray Absorption Spectroscopy (XAS) and X-ray Absorption Near Edge Spectroscopy (XANES). Via a process of elimination, we suggest that the mechanism for O redox in this material is dominated by a process that involves the formation of strongly antiferromagnetic, delocalized Mn–O states which form after Mg2+ migration at high voltages. Our results primarily rely on noninvasive techniques that are vital to understanding the electronic structure of metastable cycled cathode samples.
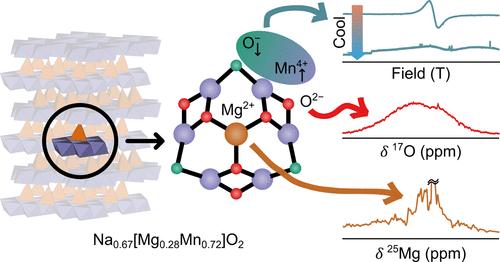
强磁交换相互作用和去局域化 Mn-O 态使 Na 离子阴极 P2-Na0.67[Mg0.28Mn0.72]O2 具有高电压容量
可充电锂离子电池和钠离子电池(分别为 LIB 和 NIB)的氧氧化还原活性正极材料所提供的更大容量,为下一代用于设备、运输和电网的高重力容量正极提供了一条途径。然而,许多此类材料都存在电压衰减、电压滞后和氧气损失等问题,其根源可追溯到循环过程中电子和化学结构的变化。详细了解这些变化对于减轻这些阴极的不良性能至关重要。在这项工作中,我们分析了 P2-Na0.67[Mg0.28Mn0.72]O2 的氧化还原机制,这是一种层状 NIB 阴极,其高容量先前被归因于捕获的 O2 分子。我们研究了各种电荷补偿方案,计算了相应的状态密度和光谱特性,并将结果与实验数据进行了系统比较:这些实验数据包括:25Mg 和 17O 核磁共振 (NMR) 光谱、操作 X 波段和原位高频电子顺磁共振 (EPR)、原位磁力计以及 O 和 Mn K 边 X 射线吸收光谱 (XAS) 和 X 射线吸收近边光谱 (XANES)。通过排除法,我们认为这种材料中的 O 氧化还原机制主要是在高电压下 Mg2+ 迁移后形成的强反铁磁性脱局域 Mn-O 态。我们的研究结果主要依赖于非侵入性技术,这些技术对于了解可循环阴极样品的电子结构至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


