Novel 18F-Labeled Benzimidazolone-Based Radioligands as Highly Selective Sigma-2 Receptor Probes for Tumor Imaging
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
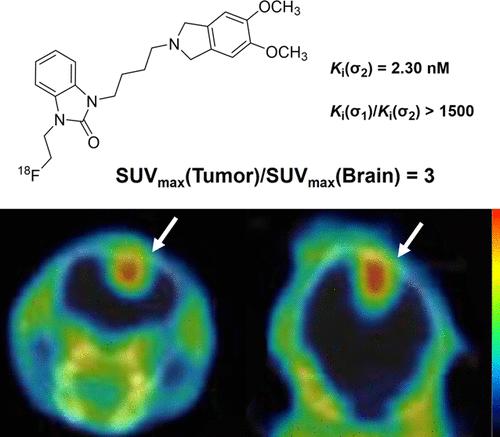
Novel sigma-2 (σ2) receptor ligands with benzimidazolone and 5,6-dimethoxyisoindoline as pharmacophores were designed and synthesized. Compound 4 exhibited low nanomolar affinity for the σ2 receptors (Ki(σ2) = 2.30 nM) and high subtype selectivity (Ki(σ1)/Ki(σ2) > 1500). Radioligand [18F]4 was prepared in radiochemical yields of 18 ± 7%, with >99% radiochemical purity and molar activity of 244 ± 136 GBq/μmol. Biodistribution and blocking studies in mice and small animal PET/CT imaging in rats indicated highly specific binding of [18F]4 in organs known to express the σ2 receptors. Small animal PET/CT imaging with [18F]4 showed clear visualization of the tumors in subcutaneous A549 lung cancer and U87MG glioma xenografts, and intracranial orthotopic U87MG glioma models. Co-administration of CM398 with [18F]4 significantly reduced activity uptake in the tumors, indicating that [18F]4 specifically binds to the σ2 receptors expressed in A549 and U87MG xenografts.
新型 18F 标记苯并咪唑酮类放射配体作为肿瘤成像的高选择性 Sigma-2 受体探针
设计并合成了以苯并咪唑酮和 5,6-二甲氧基异吲哚啉为药代体的新型 sigma-2 (σ2)受体配体。化合物 4 对 σ2 受体具有低纳摩尔亲和力(Ki(σ2) = 2.30 nM)和高亚型选择性(Ki(σ1)/Ki(σ2) >1500)。放射性配体[18F]4的放射化学收率为18 ± 7%,放射化学纯度为99%,摩尔活性为244 ± 136 GBq/μmol。小鼠的生物分布和阻断研究以及大鼠的小动物 PET/CT 成像显示,[18F]4 在已知表达 σ2 受体的器官中具有高度特异性结合。用[18F]4进行的小动物PET/CT成像显示,皮下A549肺癌和U87MG胶质瘤异种移植物以及颅内正位U87MG胶质瘤模型中的肿瘤清晰可见。CM398与[18F]4联合给药可显著降低肿瘤的活性摄取,这表明[18F]4能与A549和U87MG异种移植物中表达的σ2受体特异性结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


