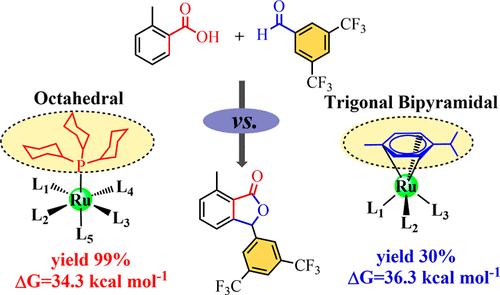
Theoretical Study on the Mechanism of Ru(II)-Catalyzed Intermolecular [3 + 2] Annulation between o-Toluic Acid and 3,5-Bis(trifluoromethyl)benzaldehyde: Octahedral vs Trigonal Bipyramidal
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Density functional theory was utilized to investigate the mechanism of Ru(II)-catalyzed aromatic C–H activation and addition of aromatic aldehydes. The proposed catalytic cycle consists of C–H bond activation, aldehyde carbonyl insertion for C–C coupling, lactonization for the formation of the final product, product separation, and catalyst recovery. Our calculations suggest that Ru(OAc)2(PCy3) (referred to as CAT) is the most favorable active catalyst, facilitating the C–H bond activation to form a five-membered ring cycloruthenium intermediate (INT2). Subsequently, the aromatic aldehyde reactant 2a enters the Ru coordination sphere, accelerating the C–C coupling and lactonization for the formation of the final product. The involvement of acetate assists in the final product separation, while INT1 re-enters the Ru coordination sphere to initiate a new catalytic cycle. Utilizing the energetic span model, the apparent activation free energy barrier was computed to be 34.3 kcal mol–1 at 443 K. Furthermore, exploration of the reaction mechanism in the absence of phosphine ligands identified Ru(OAc)2(p-cymene) as the most favorable active catalyst. The derived apparent activation free energy barrier offers a comprehensive explanation for the experimentally observed yields. Additionally, we have examined the disparities between the octahedral and trigonal bipyramidal structures of the catalysts concerning their effects on the reaction mechanisms and apparent activation free energy barriers.
Ru(II)- 催化邻甲基苯甲酸与 3,5-双三氟甲基苯甲醛分子间[3 + 2]嵌合的机理理论研究:八面体与三叉双锥体
利用密度泛函理论研究了 Ru(II) 催化芳香族 C-H 活化和芳香醛加成的机理。所提出的催化循环包括 C-H 键活化、醛羰基插入以实现 C-C 偶联、内酯化以形成最终产物、产物分离和催化剂回收。我们的计算表明,Ru(OAc)2(PCy3)(简称 CAT)是最有利的活性催化剂,可促进 C-H 键活化,形成五元环环钌中间体 (INT2)。随后,芳香醛反应物 2a 进入 Ru 配位圈,加速 C-C 偶联和内酯化,形成最终产物。醋酸的参与有助于最终产物的分离,而 INT1 则重新进入 Ru 配位球,启动新的催化循环。此外,在没有膦配体的情况下,对反应机理的探索发现 Ru(OAc)2(p-cymene) 是最有利的活性催化剂。得出的表观活化自由能障为实验观察到的产率提供了全面的解释。此外,我们还研究了催化剂八面体结构和三叉双锥体结构之间的差异对反应机理和表观活化自由能垒的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


