The Recovery of All-Metals and Fluorine Resources from Used Lithium-Ion Batteries
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Lithium-ion battery (LIB) recycling is of critical importance, but previous efforts mainly focused on recovering transition metals (TMs), while overlooking the regaining of Li-resources and the control of fluorine pollution. Here, we propose a strategy for recovering both lithium and TMs from the electrolyte and cathode of used LIBs while simultaneously addressing fluorine pollution. The recovery process involves extracting lithium and 1/6 of F from LiPF6 in the electrolyte, forming LiF at a yield of 93.0%. The remaining 5/6 F in LiPF6 is captured by NaOH and transforms into NaF in a yield of 93.9%. Different recovery routes of Li and TMs in the cathode were compared, including formic-acid leaching, and following direct recalcination, formic-acid leaching and then separating TM and Li using different precipitants. Furthermore, the recovered salts can be used in the repreparation of LiNixCoyMnzO2 (x + y + z = 1). This work presents a cost-efficient strategy for the comprehensive recovery of all-metals from used LIBs and fixation of fluorine simultaneously.
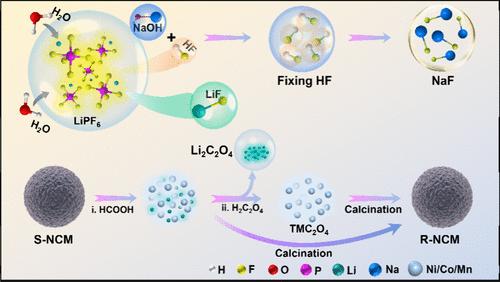
从废旧锂离子电池中回收全金属和氟资源
锂离子电池(LIB)的回收利用至关重要,但以往的工作主要集中在回收过渡金属(TMs)上,而忽略了锂资源的再利用和氟污染的控制。在此,我们提出了一种从废旧锂电池的电解液和阴极中回收锂和过渡金属的策略,同时解决氟污染问题。回收过程包括从电解液中的 LiPF6 中提取锂和 1/6 的氟,形成 LiF,产量为 93.0%。LiPF6 中剩余的 5/6 F 被 NaOH 捕获并转化为 NaF,回收率为 93.9%。比较了阴极中 Li 和 TM 的不同回收途径,包括甲酸浸出、直接再煅烧、甲酸浸出,然后使用不同的沉淀剂分离 TM 和 Li。此外,回收的盐可用于重新制备 LiNixCoyMnzO2(x + y + z = 1)。这项研究提出了一种具有成本效益的策略,可同时从废旧锂电池中全面回收所有金属并固定氟。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


