Severity Scores for Cutaneous Lupus Erythematosus
IF 5.7
2区 医学
Q1 DERMATOLOGY
引用次数: 0
Abstract
Despite the significant disease burden of cutaneous lupus erythematosus (CLE), there have been no United States Food and Drug Administration–approved therapies for 65 years. To facilitate advancement of therapies, severity scores are needed to evaluate QOL, how patients feel, activity of disease, and organ-specific damage to assess response to therapies and disease progression. In this paper, we delineate the development process of provider- and patient-reported severity scores for CLE. Cutaneous Lupus Disease Area and Severity Index (CLASI), a provider-reported measure that distinguishes between activity and damage, has undergone rigorous validation and reliability testing for over 20 years. Its performance has been tested in clinical trials as a primary or secondary endpoint and tool to stratify patients. As an objective disease measure that captures a provider’s perspective of disease activity and damage, the CLASI inherently does not assess disease impact on patients’ QOL. Cutaneous Lupus Erythematosus Quality of Life (CLEQoL), a patient-reported measure, captures features elucidated through focus groups, including symptoms, emotions, functioning, body image, and photosensitivity. It has undergone psychometric property testing to ensure reliability and validity. Together, CLASI and CLEQoL are simple and reliable CLE-specific severity scores capturing disease activity, damage, and QOL from provider and patient perspectives.
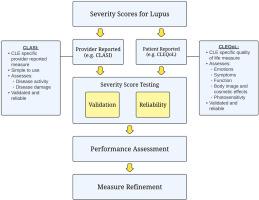
皮肤红斑狼疮的严重程度评分。
尽管皮肤红斑狼疮(CLE)给患者带来了沉重的疾病负担,但美国食品和药物管理局批准的疗法已有 65 年之久。为了促进疗法的发展,需要对严重程度进行评分,以评估患者的生活质量、患者的感受、疾病的活动性和器官特异性损伤,从而评估对疗法的反应和疾病的进展。在本文中,我们描述了由提供者和患者报告的 CLE 严重程度评分的开发过程。皮肤狼疮疾病面积和严重程度指数(CLASI)是一种由医疗服务提供者报告的测量方法,可区分活动性和损伤,20 多年来已通过了严格的验证和可靠性测试。其性能已在临床试验中作为主要或次要终点以及对患者进行分层的工具进行了测试。作为一种客观的疾病测量方法,CLASI 可捕捉医疗服务提供者对疾病活动性和损害的看法,但本质上并不评估疾病对患者 QOL 的影响。皮肤红斑狼疮患者生活质量(CLEQoL)是一项由患者报告的测量指标,它捕捉了通过焦点小组阐明的特征,包括症状、情绪、功能、身体形象和光敏感性。它经过了心理特性测试,以确保可靠性和有效性。CLASI 和 CLEQoL 是简单可靠的 CLE 严重程度评分,可从提供者和患者的角度反映疾病的活动性、损害和 QOL。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.70
自引率
4.60%
发文量
1610
审稿时长
2 months
期刊介绍:
Journal of Investigative Dermatology (JID) publishes reports describing original research on all aspects of cutaneous biology and skin disease. Topics include biochemistry, biophysics, carcinogenesis, cell regulation, clinical research, development, embryology, epidemiology and other population-based research, extracellular matrix, genetics, immunology, melanocyte biology, microbiology, molecular and cell biology, pathology, percutaneous absorption, pharmacology, photobiology, physiology, skin structure, and wound healing

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


