A viscoelastic constitutive framework for aging muscular and elastic arteries
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
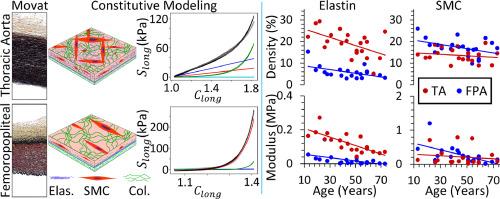
The evolution of arterial biomechanics and microstructure with age and disease plays a critical role in understanding the health and function of the cardiovascular system. Accurately capturing these adaptative processes and their effects on the mechanical environment is critical for predicting arterial responses. This challenge is exacerbated by the significant differences between elastic and muscular arteries, which have different structural organizations and functional demands. In this study, we aim to shed light to these adaptive processes by comparing the viscoelastic mechanics of autologous thoracic aortas (TA) and femoropopliteal arteries (FPA) in different age groups. We have extended our fractional viscoelastic framework, originally developed for FPA, to both types of arteries. To evaluate this framework, we analyzed experimental mechanical data from TA and FPA specimens from 21 individuals aged 13 to 73 years. Each specimen was subjected to a multi-ratio biaxial mechanical extension and relaxation test complemented by bidirectional histology to quantify the structural density and microstructural orientations. Our new constitutive model accurately captured the mechanical responses and microstructural differences of the tissues and closely matched the experimentally measured densities. It was found that the viscoelastic properties of collagen and smooth muscle cells (SMCs) in both the FPA and TA remained consistent with age, but the viscoelasticity of the SMCs in the FPA was twice that of the TA. Additionally, changes in collagen nonlinearity with age were similar in both TA and FPA. This model provides valuable insights into arterial mechanophysiology and the effects of pathological conditions on vascular biomechanics.
Statement of significance
Developing durable treatments for arterial diseases necessitates a deeper understanding of how mechanical properties evolve with age in response to mechanical environments. In this work, we developed a generalized viscoelastic constitutive model for both elastic and muscular arteries and analyzed both the thoracic aorta (TA) and the femoropopliteal artery (FPA) from 21 donors aged 13 to 73. The derived parameters correlate well with histology, allowing further examination of how viscoelasticity evolves with age. Correlation between the TA and FPA of the same donors suggest that the viscoelasticity of the FPA may be influenced by the TA, necessitating more detailed analysis. In summary, our new model proves to be a valuable tool for studying arterial mechanophysiology and exploring pathological impacts.
老化肌肉和弹性动脉的粘弹性构成框架
动脉生物力学和微观结构随年龄和疾病的演变对了解心血管系统的健康和功能起着至关重要的作用。准确捕捉这些适应过程及其对机械环境的影响对于预测动脉反应至关重要。由于弹性动脉和肌肉动脉具有不同的结构组织和功能需求,两者之间的显著差异加剧了这一挑战。在本研究中,我们通过比较不同年龄组的自体胸主动脉(TA)和股动脉(FPA)的粘弹性力学,旨在揭示这些适应过程。我们将最初针对 FPA 开发的分数粘弹性框架扩展到这两种类型的动脉。为了评估这一框架,我们分析了来自 21 个年龄在 13 到 73 岁之间的人的 TA 和 FPA 标本的实验力学数据。每个标本都进行了多比率双轴机械伸展和松弛测试,并辅以双向组织学检查,以量化结构密度和微结构取向。我们的新构成模型准确地捕捉到了组织的机械响应和微观结构差异,并与实验测量的密度非常吻合。研究发现,FPA 和 TA 中胶原蛋白和平滑肌细胞(SMC)的粘弹性随年龄增长保持一致,但 FPA 中 SMC 的粘弹性是 TA 的两倍。此外,TA 和 FPA 的胶原非线性随年龄的变化也相似。该模型为动脉机械生理学以及病理条件对血管生物力学的影响提供了宝贵的见解。意义说明:要开发出治疗动脉疾病的持久疗法,就必须更深入地了解机械特性是如何随着年龄的增长在机械环境中发生演变的。在这项工作中,我们为弹性动脉和肌肉动脉开发了一个广义粘弹性构成模型,并分析了 21 名年龄在 13 到 73 岁之间的捐献者的胸主动脉(TA)和股动脉(FPA)。得出的参数与组织学有很好的相关性,可以进一步研究粘弹性是如何随年龄演变的。同一供体的 TA 和 FPA 之间的相关性表明,FPA 的粘弹性可能受到 TA 的影响,因此有必要进行更详细的分析。总之,我们的新模型被证明是研究动脉机械生理学和探索病理影响的宝贵工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


