3D-ink-extruded titanium scaffolds with porous struts and bioactive supramolecular polymers for orthopedic implants
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Porous titanium addresses the longstanding orthopedic challenges of aseptic loosening and stress shielding. This work expands on the evolution of porous Ti with the manufacturing of hierarchically porous, low stiffness, ductile Ti scaffolds via direct-ink write (DIW) extrusion and sintering of inks containing Ti and NaCl particles. Scaffold macrochannels were filled with a subtherapeutic dose of recombinant bone morphogenetic protein-2 (rhBMP-2) alone or co-delivered within a bioactive supramolecular polymer slurry (SPS) composed of peptide amphiphile nanofibrils and collagen, creating four treatment conditions (Ti struts: microporous vs. fully dense; BMP-2 alone or with SPS). The BMP-2-loaded scaffolds were implanted bilaterally across the L4 and L5 transverse processes in a rat posterolateral lumbar fusion model. In-vivo bone growth in these scaffolds is evaluated with synchrotron X-ray computed microtomography (µCT) to study the effects of strut microporosity and added biological signaling agents on the bone formation response. Optical and scanning electron microscopy confirms the ∼100 µm space-holder micropore size, high-curvature morphology, and pore fenestrations within the struts. Uniaxial compression testing shows that the microporous strut scaffolds have low stiffness and high ductility. A significant promotion in bone formation was observed for groups utilizing the SPS, while no significant differences were found for the scaffolds with the incorporation of micropores.
Statement of significance
By 2050, the anticipated number of people aged 60 years and older worldwide is anticipated to double to 2.1 billion. This rapid increase in the geriatric population will require a corresponding increase in orthopedic surgeries and more effective materials for longer indwelling times. Titanium alloys have been the gold standard of bone fusion and fixation, but their use has longstanding limitations in bone-implant stiffness mismatch and insufficient osseointegration. We utilize 3D-printing of titanium with NaCl space holders for large- and small-scale porosity and incorporate bioactive supramolecular polymers into the scaffolds to increase bone growth. This work finds no significant change in bone ingrowth via space-holder-induced microporosity but significant increases in bone ingrowth via the bioactive supramolecular polymers in a rat posterolateral fusion model.
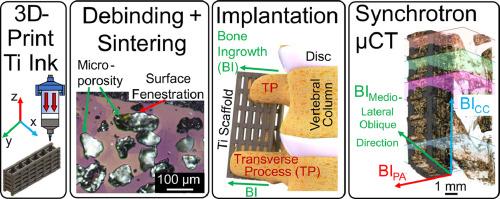
具有多孔支柱和生物活性超分子聚合物的三维墨水挤压钛支架,用于骨科植入物。
多孔钛解决了整形外科长期以来面临的无菌松动和应力屏蔽难题。这项研究拓展了多孔钛的发展,通过直接墨水写入(DIW)挤压和烧结含有钛和氯化钠颗粒的墨水,制造出分层多孔、低刚度、韧性好的钛支架。在支架大通道中单独填充亚治疗剂量的重组骨形态发生蛋白-2(rhBMP-2),或在由肽两性纳米纤维和胶原蛋白组成的生物活性超分子聚合物浆料(SPS)中共同填充重组骨形态发生蛋白-2,形成四种处理条件(钛支架:微孔与全致密;单独或与 SPS)。在大鼠后外侧腰椎融合模型中,将 BMP-2 负载支架横跨 L4 和 L5 横向椎体植入双侧。利用同步辐射 X 射线计算机显微层析技术(µCT)评估了这些支架的体内骨生长情况,以研究支柱微孔和添加的生物信号物质对骨形成反应的影响。光学显微镜和扫描电子显微镜证实了支撑杆内∼100 微米的空间夹层微孔尺寸、高曲率形态和孔隙。单轴压缩测试表明,微孔支架具有低刚度和高延展性。在使用 SPS 的组别中,骨形成有明显的促进作用,而加入微孔的支架则没有发现明显的差异。意义说明:到 2050 年,全球 60 岁及以上人口预计将翻一番,达到 21 亿。老年人口的快速增长将要求骨科手术的数量相应增加,并需要更有效的材料来延长留置时间。钛合金一直是骨融合和固定的黄金标准,但其使用长期以来存在骨-植入物刚度不匹配和骨结合不足的局限性。我们利用三维打印技术将钛与氯化钠空间支架结合,以获得大尺度和小尺度的孔隙率,并在支架中加入生物活性超分子聚合物,以增加骨生长。这项研究发现,在大鼠后外侧融合模型中,通过空间支架诱导的微孔对骨生长没有明显影响,但通过生物活性超分子聚合物对骨生长有明显增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


