Spatial analysis reveals targetable macrophage-mediated mechanisms of immune evasion in hepatocellular carcinoma minimal residual disease
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Hepatocellular carcinoma (HCC) frequently recurs from minimal residual disease (MRD), which persists after therapy. Here, we identified mechanisms of persistence of residual tumor cells using post-chemoembolization human HCC (n = 108 patients, 1.07 million cells) and a transgenic mouse model of MRD. Through single-cell high-plex cytometric imaging, we identified a spatial neighborhood within which PD-L1 + M2-like macrophages interact with stem-like tumor cells, correlating with CD8+ T cell exhaustion and poor survival. Further, through spatial transcriptomics of residual HCC, we showed that macrophage-derived TGFβ1 mediates the persistence of stem-like tumor cells. Last, we demonstrate that combined blockade of Pdl1 and Tgfβ excluded immunosuppressive macrophages, recruited activated CD8+ T cells and eliminated residual stem-like tumor cells in two mouse models: a transgenic model of MRD and a syngeneic orthotopic model of doxorubicin-resistant HCC. Thus, our spatial analyses reveal that PD-L1+ macrophages sustain MRD by activating the TGFβ pathway in stem-like cancer cells and targeting this interaction may prevent HCC recurrence from MRD. Dhanasekaran and colleagues study minimal residual disease in hepatocellular carcinoma using single-cell spatial transcriptomic and proteomic analysis and find a targetable role for immunosuppressive macrophages.
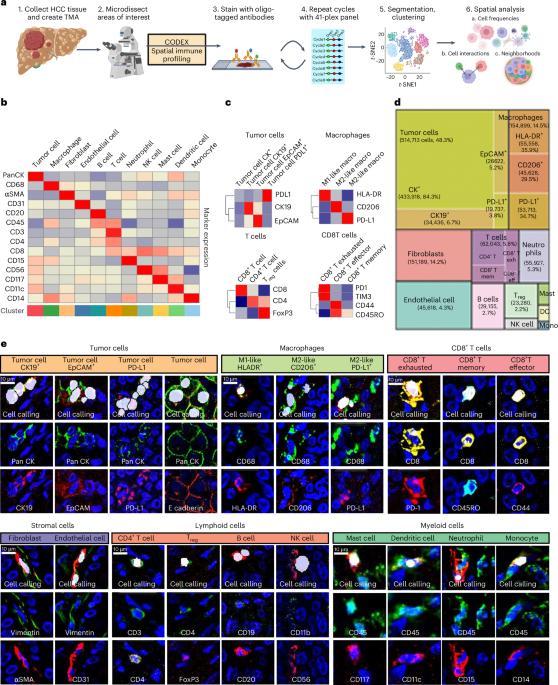
空间分析揭示了由巨噬细胞介导的肝癌极小残留病免疫逃避机制。
肝细胞癌(HCC)经常因最小残留病(MRD)而复发,MRD 在治疗后仍然存在。在这里,我们利用化疗栓塞后人类 HCC(n = 108 例患者,107 万个细胞)和 MRD 转基因小鼠模型确定了残留肿瘤细胞的持续存在机制。通过单细胞高倍细胞计量成像,我们确定了PD-L1 + M2样巨噬细胞与干样肿瘤细胞相互作用的空间邻域,该邻域与CD8+ T细胞衰竭和生存率低下相关。此外,通过残留 HCC 的空间转录组学,我们发现巨噬细胞衍生的 TGFβ1 介导了干样肿瘤细胞的持续存在。最后,我们证明了在两种小鼠模型中联合阻断 Pdl1 和 Tgfβ 可排除免疫抑制巨噬细胞、招募活化的 CD8+ T 细胞并消除残留的干样肿瘤细胞:MRD 的转基因模型和多柔比星耐药 HCC 的同种异体模型。因此,我们的空间分析表明,PD-L1+巨噬细胞通过激活干样癌细胞中的TGFβ通路来维持MRD,针对这种相互作用可防止MRD导致的HCC复发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


