A novel fungal sensor (Ngs1) of N-acetylglucosamine (GlcNAc) mediates the fungal response to GlcNAc in the interaction between entomopathogenic Beauveria bassiana and insect host
IF 3.6
3区 生物学
Q1 ZOOLOGY
引用次数: 0
Abstract
As N-acetylglucosamine (GlcNAc) ubiquitously exists in both insect cuticle and fungal cell walls, the GlcNAc sensor (Ngs1) potentially plays important roles in the interactions between entomopathogenic fungi and their insect hosts. However, the roles of the Ngs1 derived from the entomopathogens in response to the host’s cuticle remain completely unexplored. In this study, a putative Ngs1 homolog was identified in the entomopathogenic fungus Beauveria bassiana. Deletion of Ngs1 significantly reduced virulence towards Galleria mellonella larvae either through cuticle infection (by 23%) or by bypassing the cuticle (by 44%). To investigate the role of Ngs1 in fungal virulence, an analysis of the transcriptome induced by Locusta migratoria exoskeleton was conducted, highlighting the regulatory mechanism of Ngs1 in carbohydrate metabolic process, particularly chitin metabolism and GlcNAc metabolism. Consistent with the transcriptomic data, Ngs1-deletion mutants showed reduced activities of both secreted chitinase (17% reduction) and Pr1 protease (35% reduction). Loss of Ngs1 down-regulated the transcript levels of GlcNAc-catabolism genes, resulting in a 17% decrease in fungal growth on GlcNAc-supported media. Furthermore, Ngs1 deficiency attenuated the fungal response to GlcNAc, leading to the alteration of fungal resistance to diverse stress cues. All of these changes contribute to the reduction in virulence in Ngs1-deficient B. bassiana. These findings support that Ngs1 plays a critical role in responding to insect-derived GlcNAc, affecting the production of cuticle-degrading enzymes to penetrate insect epidermis, GlcNAc-induced changes of stress resistance, and contribute to the fungal virulence against insects.
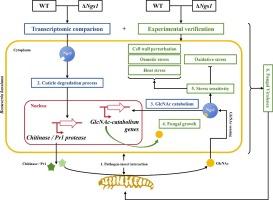
N-acetylglucosamine (GlcNAc)的新型真菌传感器(Ngs1)在昆虫病原菌Beauveria bassiana与昆虫宿主的相互作用中介导真菌对GlcNAc的反应。
由于N-乙酰葡糖胺(GlcNAc)普遍存在于昆虫角质层和真菌细胞壁中,因此GlcNAc传感器(Ngs1)可能在昆虫病原真菌与其昆虫宿主的相互作用中发挥重要作用。然而,来自昆虫病原菌的 Ngs1 在响应宿主角质层时的作用仍完全未被探索。本研究在昆虫病原真菌 Beauveria bassiana 中发现了一种推测的 Ngs1 同源物。通过角质层感染(23%)或绕过角质层(44%),Ngs1 的缺失大大降低了对 Galleria mellonella 幼虫的毒力。为了研究 Ngs1 在真菌毒力中的作用,研究人员对蝗虫外骨骼诱导的转录组进行了分析,突出了 Ngs1 在碳水化合物代谢过程中的调控机制,特别是几丁质代谢和 GlcNAc 代谢。与转录组数据一致,Ngs1缺失突变体的分泌几丁质酶(降低17%)和Pr1蛋白酶(降低35%)活性均有所降低。Ngs1 缺失会降低 GlcNAc 代谢基因的转录水平,导致真菌在 GlcNAc 支持的培养基上的生长降低 17%。此外,Ngs1 缺乏还削弱了真菌对 GlcNAc 的反应,导致真菌对各种应激线索的抗性发生改变。所有这些变化都有助于降低缺乏 Ngs1 的 B. bassiana 的毒力。这些研究结果证明,Ngs1 在响应昆虫来源的 GlcNAc 过程中起着关键作用,它影响角质层降解酶的产生以穿透昆虫表皮,影响 GlcNAc 诱导的应激抗性变化,并促进真菌对昆虫的毒力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


