Accelerated removal of solvent residuals from PLGA microparticles by alcohol vapor-assisted fluidized bed drying
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The removal of residual solvents from biodegradable poly(D,L-lactide-co-glycolide) (PLGA) microparticles by fluidized bed drying was investigated. Microparticles were prepared by the O/W solvent extraction/evaporation method and the influence of various process and formulation parameters on the secondary drying was studied. PLGA microparticles and films were characterized for residual organic solvent and water content, recrystallisation, surface morphology, drug loading and in-vitro release of the drugs dexamethasone and risperidone. While alcohol-free fluidized bed drying decreased the residual dichloromethane content only from about 7 % (w/w) to 6.4 % (w/w) (18 °C) or 3.2 % (w/w) (35 °C) within 24 h, 140 mg/L methanol vapor in purge gas facilitated almost complete removal of dichloromethane or ethyl acetate from microparticles (0–0.11 % (w/w) after 6 h). By controlling the alcohol concentration and temperature of the purge gas, the alcohol absorption and complete removal was controlled. Risperidone increased the methanol absorption enhancing the plasticization. A high initial residual water content was identified to promote aggregation and was eliminated by starting fluidized bed drying without alcohol. Alcohol vapor-assisted fluidized bed drying accelerated microparticle manufacturing without affecting the redispersibility, the drug loading and the in-vitro release of risperidone and dexamethasone.
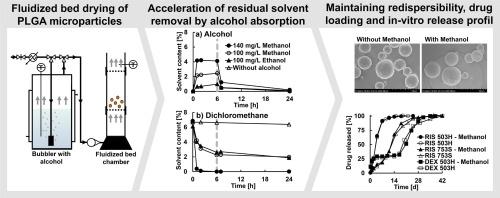
用酒精蒸汽辅助流化床干燥法加速去除聚乳酸乙烯-丙烯酸(PLGA)微粒中的溶剂残留。
研究了通过流化床干燥去除可生物降解的聚(D,L-乳酸-共聚乙二醇)(PLGA)微粒中的残留溶剂。微粒采用 O/W 溶剂萃取/蒸发法制备,研究了各种工艺和配方参数对二次干燥的影响。对 PLGA 微颗粒和薄膜的残留有机溶剂和水含量、再结晶、表面形态、药物负载以及药物地塞米松和利培酮的体外释放进行了表征。无醇流化床干燥在24小时内仅将二氯甲烷的残留量从约7%(w/w)降至6.4%(w/w)(18 °C)或3.2%(w/w)(35 °C),而净化气体中的140毫克/升甲醇蒸汽几乎能完全去除微颗粒中的二氯甲烷或乙酸乙酯(6小时后为0--0.11%(w/w))。通过控制酒精浓度和净化气体的温度,可以控制酒精的吸收和完全去除。利培酮增加了甲醇的吸收,提高了塑化效果。研究发现,高初始残余水含量会促进聚结,而在不使用酒精的情况下开始流化床干燥,则可消除残余水含量。酒精蒸汽辅助流化床干燥加速了微粒的制造,但不会影响利培酮和地塞米松的可再分散性、药物负载和体外释放。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


