Comparison of in vitro screening methods for evaluating the effects of pharmaceutical excipients on membrane permeability
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The effects of pharmaceutical excipients on intestinal drug absorption have been highlighted and careful excipient selection is required to develop biologically equivalent formulations. This study aimed to evaluate the effects of excipients on drug permeability and compare the characteristics of in vitro screening methods. Three in vitro models, the commercial precoated parallel artificial membrane permeability assay (PAMPA), PermeaPadTM, and Caco-2 monolayer, were used to evaluate the effects of 14 excipients on the permeability of several drugs with different biopharmaceutical classification system classes. Concentration-dependent effects were analyzed to distinguish non-specific effects. The permeability of low-permeability drugs was increased by excipients such as hydroxypropyl cellulose and povidone K30 in the precoated PAMPA model, whereas PermeaPadTM maintained membrane integrity at higher concentrations. Conversely, croscarmellose sodium and sodium lauryl sulfate (SLS) decreased the permeability of highly permeable drugs in both precoated PAMPA and PermeaPadTM assays in a concentration-dependent manner. In Caco-2 monolayer assays, most excipients showed minimal effects on drug permeability. However, SLS significantly reduces the permeability of highly permeable drugs at concentrations above the critical micelle concentration, thereby compromising the integrity of the cell monolayer. Our results suggested that most of excipients, except SLS, did not affect the membrane permeation of drugs at clinically used concentrations. The pre-coated PAMPA model demonstrated high sensitivity to excipient effects, making it suitable for conservative evaluation. The PermeaPadTM and Caco-2 models allowed assessment at higher excipient concentrations, with PermeaPadTM being particularly useful for excipients that cause toxicity in Caco-2 cells.
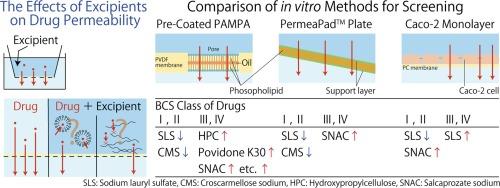
评估药用辅料对膜渗透性影响的体外筛选方法比较。
药用辅料对肠道药物吸收的影响一直备受关注,因此需要谨慎选择辅料以开发生物等效制剂。本研究旨在评估辅料对药物渗透性的影响,并比较体外筛选方法的特点。研究采用了三种体外模型,即商用预涂平行人工膜渗透性试验(PAMPA)、PermeaPadTM 和 Caco-2 单层,来评估 14 种辅料对不同生物制药分类系统类别的几种药物渗透性的影响。分析了浓度依赖性效应,以区分非特异性效应。在预包衣 PAMPA 模型中,羟丙基纤维素和聚维酮 K30 等辅料增加了低渗透性药物的渗透性,而 PermeaPadTM 在较高浓度时能保持膜的完整性。相反,在预涂 PAMPA 和 PermeaPadTM 试验中,croscarmellose sodium 和十二烷基硫酸钠(SLS)以浓度依赖的方式降低了高渗透性药物的渗透性。在 Caco-2 单层试验中,大多数辅料对药物渗透性的影响极小。然而,当浓度超过临界胶束浓度时,SLS 会明显降低高渗透性药物的渗透性,从而损害细胞单层的完整性。我们的研究结果表明,除 SLS 外,大多数辅料在临床使用浓度下都不会影响药物的膜渗透性。预涂 PAMPA 模型对辅料影响的敏感度很高,因此适用于保守评估。PermeaPadTM 和 Caco-2 模型可在辅料浓度较高时进行评估,其中 PermeaPadTM 尤其适用于对 Caco-2 细胞有毒性的辅料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


