Development and integration of a continuous horizontal belt filter into drug production procedure
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
In the pharmaceutical industry, filtration is traditionally carried out in batch mode. However, with the spread of continuous technologies, there is an increasing demand for robust continuous filtration strategies suitable for processing suspensions produced in continuous crystallizers. Accordingly, this study aimed to investigate a lab-scale horizontal conveyor belt filtration approach for pharmaceutical separation purposes for the first time. The newly developed continuous horizontal belt filter (CHBF) was tested under different systems (microcrystalline cellulose (MCC)/water, lactose/ethanol and acetylsalicylic acid (ASA)/water) and diverse conditions. Filtration was robust using a well-defined unimodal particle size distribution MCC in water system, where the residual moisture content varied within narrow limits of 45–52% independently from the process conditions. Besides, the residual moisture content highly depended on the applied solvent and particle size. It could be reduced to below 2% by processing the suspensions of either a volatile solvent (lactose in ethanol) or an aqueous slurry of a large particle size ASA. Finally, the CHBF was connected to a mixed suspension mixed product removal (MSMPR) or a plug flow crystallizer (PFC). The residual moisture content of the CHBF-filtered ASA product and operation characteristics (onset of steady-state) were evaluated in both continuous crystallizer-filter systems. The MSMPR-CHBF system operated with a longer startup period. The size of the in situ-produced crystals was of a similar order magnitude in both systems, resulting in a similar residual moisture content (around 20%). Overall, the tested continuous filter was robust, did not modify the crystal morphology in the examined experimental range, and could be effectively integrated with continuous crystallizers.
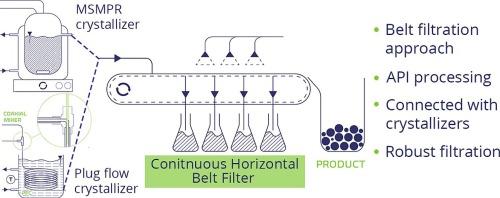
开发连续式水平带式过滤机并将其纳入药品生产程序。
在制药行业,过滤传统上是以间歇模式进行的。然而,随着连续技术的普及,对适用于处理连续结晶器生产的悬浮液的强大连续过滤策略的需求日益增加。因此,本研究旨在首次研究一种用于制药分离的实验室规模水平传送带过滤方法。在不同系统(微晶纤维素(MCC)/水、乳糖/乙醇和乙酰水杨酸(ASA)/水)和不同条件下,对新开发的连续水平带式过滤器(CHBF)进行了测试。在水体系中使用明确的单模态粒度分布的 MCC,过滤效果很好,残余水分含量在 45-52% 的狭窄范围内变化,不受工艺条件的影响。此外,残余水分含量在很大程度上取决于所使用的溶剂和颗粒大小。通过处理挥发性溶剂(乙醇中的乳糖)悬浮液或大粒径 ASA 的水性浆液,可将残余水分含量降至 2% 以下。最后,将 CHBF 连接到混合悬浮液混合产品去除装置 (MSMPR) 或塞流结晶器 (PFC)。在这两种连续结晶器-过滤器系统中,对经 CHBF 过滤的 ASA 产品的残余水分含量和运行特性(稳态开始)进行了评估。MSMPR-CHBF 系统的启动时间较长。两个系统原位生成的晶体大小相近,因此残余水分含量也相近(约 20%)。总之,测试的连续式过滤器性能稳定,在实验范围内不会改变晶体形态,并能与连续式结晶器有效集成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


