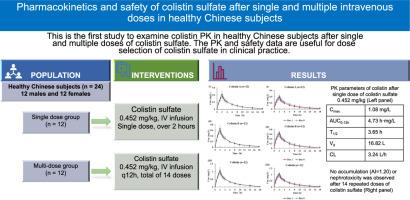
Pharmacokinetics and safety of colistin sulfate after single and multiple intravenous doses in healthy Chinese subjects
IF 4.9
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2024-09-12
DOI:10.1016/j.ijantimicag.2024.107326
引用次数: 0
Abstract
Objective
Increasing antimicrobial resistance has led to the revival of the polymyxins as a last-resort therapeutic option for multidrug-resistant Gram-negative bacterial infections. A parenteral formulation of colistin sulfate is available solely in China. While the onset of action of IV colistin may occur faster than with its prodrug CMS, its pharmacokinetic (PK) profile remains unclear.
Methods
This single-centre, open-label, single- and multi-dose, phase 1 trial examined the PKs and safety of colistin sulfate in healthy Chinese adults. Participants received a single 10,000 units/kg (equivalent to 0.452 mg/kg) dose of colistin sulfate (single-dose group, n = 12) or the same dose q12h for 7 days (multi-dose group, n = 12) via a 2-h IV infusion. Colistin concentrations in plasma and urine were determined using LC-MS/MS, and the PK parameters calculated using non-compartmental analysis.
Results
After a single dose the peak concentration (Cmax), area under the curve from 0 to 12 h (AUC0-12h), terminal half-life (T1/2), volume of distribution (Vd), and total body clearance (CL) of colistin were 1.08 ± 0.18 mg/L, 4.73 ± 0.89 h·mg/L, 3.65 ± 0.55 h, 16.82 ± 2.70 L, and 3.24 ± 0.51 L/h, respectively. No accumulation of colistin was observed after multiple doses. The cumulative urinary recovery of colistin was 0.9 ± 0.7% within 24 h after multi-dose administration. No nephrotoxicity was reported.
Conclusions
This study is the first to report colistin PKs in healthy Chinese subjects after single and multiple doses of colistin sulfate. The PK and safety data are required for optimal dose selection in clinical practice.
中国健康受试者单次和多次静脉注射硫酸可乐定的药代动力学和安全性。
背景:抗菌药耐药性的不断增加导致多粘菌素类药物作为治疗耐多药革兰氏阴性菌感染的最后选择而重新兴起。硫酸可乐定的肠外制剂仅在中国有售。虽然静脉注射可乐定的起效时间可能比其原药 CMS 更快,但其药代动力学(PK)特征仍不清楚:这项单中心、开放标签、单剂量和多剂量的 1 期试验研究了硫酸可乐定在中国健康成人中的药代动力学和安全性。参试者通过2小时静脉输注接受单剂量10000单位/千克(相当于0.452毫克/千克)的硫酸可乐定(单剂量组,12人)或连续7天每12小时接受相同剂量的硫酸可乐定(多剂量组,12人)。使用 LC-MS/MS 测定血浆和尿液中的秋水仙素浓度,并使用非室分析法计算 PK 参数:单次给药后,可乐定的峰值浓度(Cmax)、0-12 h曲线下面积(AUC0-12h)、终末半衰期(T1/2)、分布容积(Vd)和全身清除率(CL)分别为 1.08 ± 0.18 mg/L、4.73 ± 0.89 h-mg/L、3.65 ± 0.55 h、16.82 ± 2.70 L 和 3.24 ± 0.51 L/h。多次给药后未观察到可乐定蓄积。多剂量给药后 24 小时内,可乐定的累积尿回收率为 0.9 ± 0.7%。无肾毒性报告:本研究首次报道了中国健康受试者单次和多次服用硫酸秋司汀后的秋司汀PK。结论:该研究首次报道了中国健康受试者单次和多次服用硫酸可乐定后的 PK 和安全性数据,为临床实践中选择最佳剂量提供了依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


