Anticancer agent 5-fluorouracil reverses meropenem resistance in carbapenem-resistant Gram-negative pathogens
IF 4.9
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2024-09-16
DOI:10.1016/j.ijantimicag.2024.107337
引用次数: 0
Abstract
The global increasing incidence of clinical infections caused by carbapenem-resistant Gram-negative pathogens requires urgent and effective treatment strategies. Antibiotic adjuvants represent a promising approach to enhance the efficacy of meropenem against carbapenem-resistant bacteria. This study shows that the anticancer agent 5-fluorouracil (5-FU, 50 µM) significantly reduced the minimum inhibitory concentration of meropenem against blaNDM-5 positive Escherichia coli by 32-fold through cell-based high-throughput screening. Further pharmacological studies indicated that 5-FU exhibited potentiation effects on carbapenem antibiotics against 42 Gram-negative bacteria producing either metallo-β-lactamases (MBLs), such as NDM and IMP, or serine β-lactamases (Ser-BLs), like KPC and OXA. These bacteria included E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter spp., 32 of which were obtained from human clinical samples. Mechanistic investigations revealed that 5-FU inhibited the transcription and expression of the blaNDM-5 gene. In addition, 5-FU combined with meropenem enhanced bacterial metabolism, and stimulated the production of reactive oxygen species (ROS), thereby rendering bacteria more susceptible to meropenem. In a mouse systemic infection model, 5-FU combined with meropenem reduced bacterial loads and effectively elevated the survival rate of 83.3%, compared with 16.7% with meropenem monotherapy. Collectively, these findings indicate the potential of 5-FU as a novel meropenem adjuvant to improve treatment outcomes against infections caused by carbapenem-resistant bacteria.
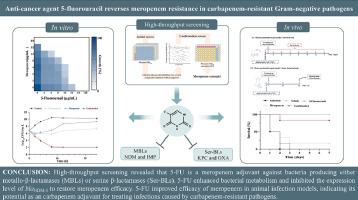
抗癌剂 5-氟尿嘧啶可逆转耐碳青霉烯类革兰氏阴性病原体对美罗培南的耐药性。
耐碳青霉烯类革兰氏阴性病原体引起的临床感染发病率在全球范围内不断上升,迫切需要有效的治疗策略。抗生素辅助剂是提高美罗培南对耐碳青霉烯类细菌疗效的一种可行方法。在此,我们通过基于细胞的高通量筛选,发现抗癌剂 5-氟尿嘧啶(5-FU,50 µM)能将美罗培南对 blaNDM-5 阳性大肠杆菌的最小抑菌浓度显著降低 32 倍。进一步的药理学研究表明,5-FU 对 42 种产生金属-β-内酰胺酶(MBLs)(如 NDM 和 IMP)或丝氨酸 β-内酰胺酶(Ser-BLs)(如 KPC 和 OXA)的革兰氏阴性细菌具有碳青霉烯类抗生素的增效作用。这些细菌包括大肠杆菌、肺炎克雷伯菌、铜绿假单胞菌和不动杆菌,其中 32 种细菌来自人类临床样本。机理研究发现,5-FU 可抑制 blaNDM-5 基因的转录和表达水平。此外,5-FU 与美罗培南合用可增强细菌的新陈代谢,刺激活性氧(ROS)的产生,从而使细菌更易受美罗培南的影响。在小鼠全身感染模型中,与美罗培南单药治疗相比,这种药物组合能有效地将存活率从 16.7% 提高到 83.3%,并减少组织中的细菌数量。总之,这些发现揭示了 5-FU 作为一种新型美罗培南辅助药物改善耐碳青霉烯类细菌感染治疗效果的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


