Estrogen, via ESR2 receptor, prevents oxidative stress-induced Müller cell death and stimulates FGF2 production independently of NRF2, attenuating retinal degeneration
IF 3
2区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
Abstract
In this study, we aimed to investigate the effects of the deficient antioxidative gene, nuclear factor-erythroid 2-related factor 2 (Nrf2), on 17β-estradiol (E2)-mediated oxidative stress response, with a specific focus on growth factor production and cell death in Müller cells and retinal tissue. Administration of hydrogen peroxide (H2O2) reduced the viability of Müller cells derived from Nrf2 wild-type (WT) and knockout (KO) mice. However, this effect was more significant in the KO cells than in the WT cells. Pretreatment with E2 inhibited H2O2-induced cell death in both Nrf2 WT and KO Müller cell genotypes. Small interfering RNA-mediated gene silencing of estrogen receptor 2 (Esr2) attenuated the cell survival-promoting activity of E2 in Nrf2 KO Müller cells, while other identified estrogen receptors, Esr1 or G protein-coupled estrogen receptor 1 (Gper1), had no effect. Western blotting revealed higher ESR2 expression levels in Nrf2 KO cells than in WT Müller cells. Conditioned media from E2-and H2O2-treated Nrf2 WT or KO Müller cells enhanced the dissociated retinal cell viability compared with H2O2-treated cells. Both quantitative reverse-transcription polymerase chain reaction assay (qRT-PCR) and enzyme-linked immunosorbent assay exhibited a significant increase in fibroblast growth factor 2 (FGF2) expression levels in E2-and H2O2-treated Nrf2 WT and KO Müller cells compared to those in E2-treated cells. In vivo, administration of N-methyl-N-nitrosourea (MNU) reduced the thickness and cell density of the outer nuclear layer (ONL) in Nrf2 KO mice and enhanced the number of terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive cells in the ONL. However, E2 administration attenuated these defects in MNU-treated mice. Concomitant administration of MNU and E2 enhanced FGF2 protein levels in retinal lysates of Nrf2 KO mice. In conclusion, E2 demonstrated a significant role in preventing oxidative stress-induced retinal cell death by stimulating FGF2 production in Müller cells, independent of the Nrf2 gene. Based on these findings, we anticipate that exogenous administration of estrogens or ESR2-selective agonists could aid in treating patients with oxidative stress-related retinal degenerative diseases such as age-related macular degeneration and retinitis pigmentosa.
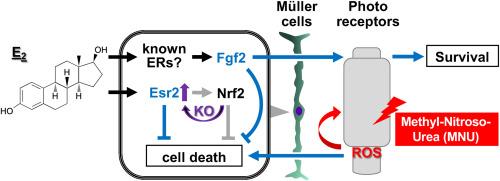
雌激素通过 ESR2 受体防止氧化应激诱导的 Müller 细胞死亡并刺激 FGF2 的产生,从而减轻视网膜退化。
在这项研究中,我们旨在研究核因子-红细胞2相关因子2(Nrf2)抗氧化基因缺陷对17β-雌二醇(E2)介导的氧化应激反应的影响,特别关注Müller细胞和视网膜组织中生长因子的产生和细胞死亡。施用过氧化氢(H2O2)会降低来自Nrf2野生型(WT)和基因敲除(KO)小鼠的Müller细胞的活力。然而,这种影响在 KO 细胞中比在 WT 细胞中更为明显。在 Nrf2 WT 和 KO Müller 细胞基因型中,用 E2 预处理都能抑制 H2O2 诱导的细胞死亡。小干扰 RNA 介导的雌激素受体 2(Esr2)基因沉默减弱了 Nrf2 KO Müller 细胞中 E2 促进细胞存活的活性,而其他已确定的雌激素受体 Esr1 或 G 蛋白偶联雌激素受体 1(Gper1)则没有影响。Western 印迹显示,Nrf2 KO 细胞中 ESR2 的表达水平高于 WT Müller 细胞。与 H2O2 处理的细胞相比,E2-和 H2O2 处理的 Nrf2 WT 或 KO Müller 细胞的条件培养基提高了离体视网膜细胞的活力。定量反转录聚合酶链反应测定(qRT-PCR)和酶联免疫吸附测定均显示,与E2处理的细胞相比,E2-和H2O2处理的Nrf2 WT和KO Müller细胞中成纤维细胞生长因子2(FGF2)的表达水平显著增加。在体内,施用N-甲基-N-亚硝基脲(MNU)会降低Nrf2 KO小鼠核外层(ONL)的厚度和细胞密度,并增加ONL中末端脱氧核苷酸转移酶dUTP缺口标记阳性细胞的数量。然而,服用E2可减轻MNU处理小鼠的这些缺陷。同时给予 MNU 和 E2 会提高 Nrf2 KO 小鼠视网膜裂解液中的 FGF2 蛋白水平。总之,E2 通过刺激 Müller 细胞中 FGF2 的产生,在防止氧化应激诱导的视网膜细胞死亡方面发挥了重要作用,这与 Nrf2 基因无关。基于这些发现,我们预计外源性服用雌激素或 ESR2 选择性激动剂有助于治疗与氧化应激相关的视网膜退行性疾病,如老年性黄斑变性和视网膜色素变性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental eye research
医学-眼科学
CiteScore
6.80
自引率
5.90%
发文量
323
审稿时长
66 days
期刊介绍:
The primary goal of Experimental Eye Research is to publish original research papers on all aspects of experimental biology of the eye and ocular tissues that seek to define the mechanisms of normal function and/or disease. Studies of ocular tissues that encompass the disciplines of cell biology, developmental biology, genetics, molecular biology, physiology, biochemistry, biophysics, immunology or microbiology are most welcomed. Manuscripts that are purely clinical or in a surgical area of ophthalmology are not appropriate for submission to Experimental Eye Research and if received will be returned without review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


