Dosage by design – 3D printing individualized cabozantinib tablets with immediate release
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-09-13
DOI:10.1016/j.ejpb.2024.114501
引用次数: 0
Abstract
Production of patient-specific dosage forms is important to improve patient adherence and effectiveness while reducing the prevalence and severity of adverse effects. Due to its possibility of rapid prototyping 3D printing can be used to produce individual dosages while utilizing techniques such as hot melt extrusion to increase the bioavailability of poorly soluble drugs. In this work, Parteck MXP and Kollicoat IR were used as water-soluble polymer bases for formulation development for 3D printing of various dosages incorporating cabozantinib while enabling immediate release. The effect of tablet design and the excipients sorbitol, croscarmellose sodium, and sodium starch glycolate was investigated for this goal. A way to calculate the size of tablets for predetermined dosages is proposed to enable the printing of individual strengths from one formulation. Rheological data were collected to deepen the understanding of the role of melt viscosity in 3D printing and hot melt extrusion processes. The production of immediate-release cabozantinib tablets containing every therapeutically relevant dosage in a single unit produced by two-step 3D printing was realized.
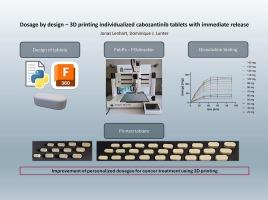
剂量设计--3D 打印可立即释放的个性化卡博替尼片。
生产针对患者的剂型对于提高患者的依从性和有效性,同时降低不良反应的发生率和严重程度非常重要。由于可以快速制作原型,3D 打印技术可用于生产个性化剂型,同时利用热熔挤出等技术提高溶解性差的药物的生物利用率。在这项工作中,Parteck MXP 和 Kollicoat IR 被用作水溶性聚合物基料,用于制剂开发,以 3D 打印出含卡博替尼的各种剂量,同时实现立即释放。为实现这一目标,研究了片剂设计和辅料山梨糖醇、croscarmellose sodium 和淀粉乙醇酸钠的影响。此外,还提出了一种计算预定剂量片剂大小的方法,以便用一种配方印制不同强度的片剂。收集了流变学数据,以加深了解熔体粘度在三维打印和热熔挤压过程中的作用。通过两步三维打印技术,实现了在单个单元中生产出包含每种治疗相关剂量的卡博替尼速释片剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


