A novel sodium caseinate lipid-based auto-emulsifying delivery system to increase resveratrol intestinal permeation: Characterization and in vitro assessment
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
In recent years, nutraceuticals have emerged as a promising strategy for maintaining health and represent a high-growth market in Italy and across Europe. However, the lack of strict regulations regarding formulation requirements and proof of efficacy raises serious concerns about their poor bioavailability and, consequently, their uncertain health benefits. An emblematic example is t-resveratrol (RES), a cardioprotective stilbene polyphenol that undergoes extensive metabolism in the intestine and liver, resulting in a bioavailability of <1 %.
This manuscript describes a novel technological matrix developed with the primary goal of improving RES oral bioavailability. This technology can be classified as a lipid-based autoemulsifying drug delivery system (LIBADDS), in which RES is thoroughly solubilized in a hot liquid phase composed of lipids and surfactants, and the mixture is further adsorbed onto a powder composed of polysaccharides and sodium caseinate (NaC), along with inert excipients, and then compressed.
In this study, NaC was used for the first time to trigger pancreatin-mediated hydrolysis of an enteric-coated tablet, allowing micellar delivery of RES to the small intestine. The RES-containing tablets were characterized via differential scanning calorimetry (DSC) and X-ray diffraction (PXRD). The digested formulation, with simulated gastric and enteric fluids, was dimensionally assessed via dynamic light scattering (DLS). Finally, calculations of the bioaccessible fraction, dissolution tests, and in vitro permeability experiments using Caco-2 cell monolayers were carried out to preliminarily define the overall efficiency and applicability of this new technology in improving RES intestinal permeability.
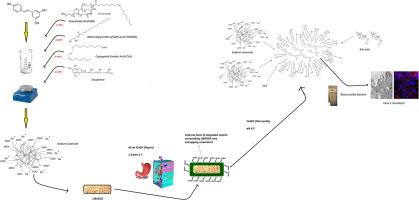
一种基于酪蛋白酸钠脂质的新型自动乳化给药系统,可增加白藜芦醇的肠道渗透性:表征和体外评估。
近年来,营养保健品已成为一种前景广阔的保健策略,在意大利和整个欧洲都是一个高速增长的市场。然而,由于缺乏有关配方要求和功效证明的严格规定,人们对其生物利用率低以及由此带来的健康益处不确定表示严重关切。一个典型的例子是 t-白藜芦醇(RES),它是一种具有心脏保护作用的二苯乙烯多酚,在肠道和肝脏中经过大量代谢,生物利用率不到 1%。本手稿介绍了一种新型技术基质,其主要目标是提高 RES 的口服生物利用率。该技术可归类为脂基自乳化给药系统(LIBADDS),其中 RES 被彻底溶解在由脂质和表面活性剂组成的热液相中,混合物被进一步吸附在由多糖和酪蛋白酸钠(NaC)以及惰性辅料组成的粉末上,然后被压缩。本研究首次使用 NaC 触发胰蛋白酶介导的肠溶片水解,从而将 RES 以胶束形式输送到小肠。含 RES 的片剂通过差示扫描量热法(DSC)和 X 射线衍射法(PXRD)进行了表征。通过动态光散射(DLS)技术,对含有模拟胃液和肠液的消化配方进行了尺寸评估。最后,还进行了生物可及部分的计算、溶解试验以及使用 Caco-2 细胞单层进行的体外渗透性实验,以初步确定这项新技术在改善 RES 肠道渗透性方面的总体效率和适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


